Radioisotopes in Medicine
(Updated April 2013)
http://www.world-nuclear.org/info/Non-Power-Nuclear-Applications/Radioisotopes/Radioisotopes-in-Medicine/#.UhbQMH-N6Sp
- Nuclear medicine uses radiation to provide diagnostic information about the functioning of a person's specific organs, or to treat them. Diagnostic procedures are now routine.
- Radiotherapy can be used to treat some medical conditions, especially cancer, using radiation to weaken or destroy particular targeted cells.
- Tens of millions of nuclear medicine procedures are performed each year, and demand for radioisotopes is increasing rapidly.
Nuclear Medicine
This is a branch of medicine that uses radiation to
provide information about the functioning of a person's specific organs
or to treat disease. In most cases, the information is used by
physicians to make a quick, accurate diagnosis of the patient's illness.
The thyroid, bones, heart, liver and many other organs can be easily
imaged, and disorders in their function revealed. In some cases
radiation can be used to treat diseased organs, or tumours. Five Nobel
Laureates have been intimately involved with the use of radioactive
tracers in medicine.
Over 10,000 hospitals worldwide use radioisotopes in medicine, and
about 90% of the procedures are for diagnosis. The most common
radioisotope used in diagnosis is technetium-99, with some 30 million
procedures per year, accounting for 80% of all nuclear medicine
procedures worldwide.
In developed countries (26% of world population) the frequency of
diagnostic nuclear medicine is 1.9% per year, and the frequency of
therapy with radioisotopes is about one tenth of this. In the USA there
are some 18 million nuclear medicine procedures per year among 311
million people, and in Europe about 10 million among 500 million people.
In Australia there are about 560,000 per year among 21 million people,
470,000 of these using reactor isotopes. The use of radiopharmaceuticals
in diagnosis is growing at over 10% per year.
Nuclear medicine was developed in the 1950s by physicians with an
endocrine emphasis, initially using iodine-131 to diagnose and then
treat thyroid disease. In recent years specialists have also come from
radiology, as dual CT/PET procedures have become established.
Computed X-ray tomography (CT) scans and nuclear medicine contribute
36% of the total radiation exposure and 75% of the medical exposure to
the US population, according to a US National Council on Radiation
Protection & Measurements report in 2009. The report showed that
Americans’ average total yearly radiation exposure had increased from
3.6 millisievert to 6.2 mSv per year since the early 1980s, due to
medical-related procedures. (Industrial radiation exposure, including
that from nuclear power plants, is less than 0.1% of overall public
radiation exposure.)
Diagnostic techniques in nuclear medicine
Diagnostic techniques in nuclear medicine use radioactive tracers
which emit gamma rays from within the body. These tracers are generally
short-lived isotopes linked to chemical compounds which permit specific
physiological processes to be scrutinised. They can be given by
injection, inhalation or orally. The first type are where single photons
are detected by a gamma camera which can view organs from many
different angles. The camera builds up an image from the points from
which radiation is emitted; this image is enhanced by a computer and
viewed by a physician on a monitor for indications of abnormal
conditions.
A more recent development is Positron Emission Tomography
(PET) which is a more precise and sophisticated technique using
isotopes produced in a cyclotron. A positron-emitting radionuclide is
introduced, usually by injection, and accumulates in the target tissue.
As it decays it emits a positron, which promptly combines with a nearby
electron resulting in the simultaneous emission of two identifiable
gamma rays in opposite directions. These are detected by a PET camera
and give very precise indication of their origin. PET's most important
clinical role is in oncology, with fluorine-18 as the tracer, since it
has proven to be the most accurate non-invasive method of detecting and
evaluating most cancers. It is also well used in cardiac and brain
imaging.
New procedures combine PET with computed X-ray tomography (CT) scans to give co-registration of the two images (PETCT),
enabling 30% better diagnosis than with traditional gamma camera alone.
It is a very powerful and significant tool which provides unique
information on a wide variety of diseases from dementia to
cardiovascular disease and cancer (oncology).
Positioning of the radiation source within the body makes the
fundamental difference between nuclear medicine imaging and other
imaging techniques such as x-rays. Gamma imaging by either method
described provides a view of the position and concentration of the
radioisotope within the body. Organ malfunction can be indicated if the
isotope is either partially taken up in the organ (cold spot), or taken
up in excess (hot spot). If a series of images is taken over a period of
time, an unusual pattern or rate of isotope movement could indicate
malfunction in the organ.
A distinct advantage of nuclear imaging over x-ray techniques is that
both bone and soft tissue can be imaged very successfully. This has led
to its common use in developed countries where the probability of
anyone having such a test is about one in two and rising.
The mean effective dose is 4.6 mSv per diagnostic procedure.
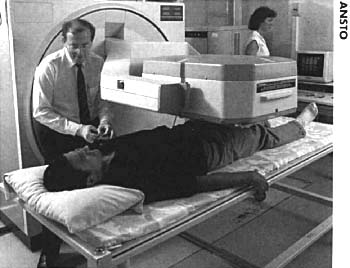
Radionuclide therapy (RNT)
Rapidly dividing cells are particularly sensitive to damage by
radiation. For this reason, some cancerous growths can be controlled or
eliminated by irradiating the area containing the growth.
External irradiation (sometimes called teletherapy) can be carried
out using a gamma beam from a radioactive cobalt-60 source, though in
developed countries the much more versatile linear accelerators are now
being utilised as a high-energy x-ray source (gamma and x-rays are much
the same). An external radiation procedure is known as the gamma knife
radiosurgery, and involves focusing gamma radiation from 201 sources of
cobalt-60 sources on a precise area of the brain with a cancerous
tumour. Worldwide, over 30,000 patients are treated annually, generally
as outpatients.
Internal radionuclide therapy is by administering or planting a small
radiation source, usually a gamma or beta emitter, in the target area.
Short-range radiotherapy is known as brachytherapy, and this is becoming
the main means of treatment. Iodine-131 is commonly used to treat
thyroid cancer, probably the most successful kind of cancer treatment.
It is also used to treat non-malignant thyroid disorders. Iridium-192
implants are used especially in the head and breast. They are produced
in wire form and are introduced through a catheter to the target area.
After administering the correct dose, the implant wire is removed to
shielded storage. This brachytherapy (short-range) procedure gives less
overall radiation to the body, is more localised to the target tumour
and is cost effective.
Treating leukaemia may involve a bone marrow transplant, in which
case the defective bone marrow will first be killed off with a massive
(and otherwise lethal) dose of radiation before being replaced with
healthy bone marrow from a donor.
Many therapeutic procedures are palliative, usually to relieve pain.
For instance, strontium-89 and (increasingly) samarium 153 are used for
the relief of cancer-induced bone pain. Rhenium-186 is a newer product
for this.
A new field is Targeted Alpha Therapy (TAT) or alpha radioimmunotherapy,
especially for the control of dispersed cancers. The short range of
very energetic alpha emissions in tissue means that a large fraction of
that radiative energy goes into the targeted cancer cells, once a
carrier such as a monoclonal antibody has taken the alpha-emitting
radionuclide to exactly the right place. Laboratory studies are
encouraging and clinical trials for leukaemia, cystic glioma and
melanoma are under way. TAT using lead-212 is said to show promise for
treating pancreatic, ovarian and melanoma cancers.
An experimental development of this is Boron Neutron Capture Therapy
using boron-10 which concentrates in malignant brain tumours. The
patient is then irradiated with thermal neutrons which are strongly
absorbed by the boron, producing high-energy alpha particles which kill
the cancer. This requires the patient to be brought to a nuclear
reactor, rather than the radioisotopes being taken to the patient.
Radionuclide therapy has progressively become successful in treating
persistent disease and doing so with low toxic side-effects. With any
therapeutic procedure the aim is to confine the radiation to
well-defined target volumes of the patient. The doses per therapeutic
procedure are typically 20-60 Gy.
Biochemical Analysis
It is very easy to detect the presence or absence of some radioactive
materials even when they exist in very low concentrations.
Radioisotopes can therefore be used to label molecules of biological
samples in vitro (out of the body). Pathologists have devised
hundreds of tests to determine the constituents of blood, serum, urine,
hormones, antigens and many drugs by means of associated radioisotopes.
These procedures are known as radioimmuno-assays and, although the
biochemistry is complex, kits manufactured for laboratory use are very
easy to use and give accurate results. In Europe some 15 million of
these in vitro analyses are undertaken each year.
Diagnostic Radiopharmaceuticals
Every organ in our bodies acts differently from a chemical point of
view. Doctors and chemists have identified a number of chemicals which
are absorbed by specific organs. The thyroid, for example, takes up
iodine, the brain consumes quantities of glucose, and so on. With this
knowledge, radiopharmacists are able to attach various radioisotopes to
biologically active substances. Once a radioactive form of one of these
substances enters the body, it is incorporated into the normal
biological processes and excreted in the usual ways.
Diagnostic radiopharmaceuticals can be used to examine blood flow to
the brain, functioning of the liver, lungs, heart or kidneys, to assess
bone growth, and to confirm other diagnostic procedures. Another
important use is to predict the effects of surgery and assess changes
since treatment.
The amount of the radiopharmaceutical given to a patient is just
sufficient to obtain the required information before its decay. The
radiation dose received is medically insignificant. The patient
experiences no discomfort during the test and after a short time there
is no trace that the test was ever done. The non-invasive nature of this
technology, together with the ability to observe an organ functioning
from outside the body, makes this technique a powerful diagnostic tool.
A radioisotope used for diagnosis must emit gamma rays of sufficient
energy to escape from the body and it must have a half-life short enough
for it to decay away soon after imaging is completed.
The radioisotope most widely used in medicine is technetium-99m,
employed in some 80% of all nuclear medicine procedures - hence some 30
million per year, of which 6-7 million are in Europe, 15 million in
North America, 6-8 million in Asia/Pacific (particularly Japan), and 0.5
million in other regions. It is an isotope of the artificially-produced
element technetium and it has almost ideal characteristics for a
nuclear medicine scan. These are:
- It has a half-life of six hours which is long enough to examine metabolic processes yet short enough to minimise the radiation dose to the patient.
- Technetium-99m decays by a process called "isomeric"; which emits gamma rays and low energy electrons. Since there is no high-energy beta emission the radiation dose to the patient is low.
- The low energy gamma rays it emits easily escape the human body and are accurately detected by a gamma camera. Once again the radiation dose to the patient is minimised.
- The chemistry of technetium is so versatile it can form tracers by being incorporated into a range of biologically-active substances to ensure that it concentrates in the tissue or organ of interest.
Its logistics also favour its use. Technetium generators, a lead pot
enclosing a glass tube containing the radioisotope, are supplied to
hospitals from the nuclear reactor where the isotopes are made. They
contain molybdenum-99, with a half-life of 66 hours, which progressively
decays to technetium-99. The Tc-99 is washed out of the lead pot by
saline solution when it is required. After two weeks or less the
generator is returned for recharging.
A similar generator system is used to produce rubidium-82 for PET imaging from strontium-82 - which has a half-life of 25 days.
Myocardial Perfusion Imaging (MPI) uses thallium-201 chloride or
technetium-99m and is important for detection and prognosis of coronary
artery disease.
Canadian 2006 data shows that 56% of Tc-99 use there is in myocardial
ischemia perfusion, 17% in bone scans, 7% in liver/hepatobiliary, 4%
respiratory, 3% renal, 3% thyroid.
For PET imaging, the main radiopharmaceutical is Fluoro-deoxy glucose
(FDG) incorporating F-18 - with a half-life of just under two hours, as
a tracer. The FDG is readily incorporated into the cell without being
broken down, and is a good indicator of cell metabolism.
In diagnostic medicine, there is a strong trend to using more
cyclotron-produced isotopes such as F-18 as PET and CT/PET become more
widely available. However, the procedure needs to be undertaken within
two hours reach of a cyclotron, which limits their utility compared with
Mo/Tc-99.
Therapeutic Radiopharmaceuticals
For some medical conditions, it is useful to destroy or weaken
malfunctioning cells using radiation. The radioisotope that generates
the radiation can be localised in the required organ in the same way it
is used for diagnosis - through a radioactive element following its
usual biological path, or through the element being attached to a
suitable biological compound. In most cases, it is beta radiation which
causes the destruction of the damaged cells. This is radionuclide
therapy (RNT) or radiotherapy. Short-range radiotherapy is known as
brachytherapy, and this is becoming the main means of treatment.
Although radiotherapy is less common than diagnostic use of
radioactive material in medicine, it is nevertheless widespread,
important and growing. An ideal therapeutic radioisotope is a strong
beta emitter with just enough gamma to enable imaging, eg lutetium-177. This is prepared from ytterbium-176 which is irradiated to become Yb-177 which decays rapidly to Lu-177. Yttrium-90
is used for treatment of cancer, particularly non-Hodgkin's lymphoma,
and its more widespread use is envisaged, including for arthritis
treatment. Lu-177 and Y-90 are becoming the main RNT agents.
Iodine-131 and phosphorus-32 are also used for therapy. Iodine-131 is
used to treat the thyroid for cancers and other abnormal conditions
such as hyperthyroidism (over-active thyroid). In a disease called
Polycythemia vera, an excess of red blood cells is produced in the bone
marrow. Phosphorus-32 is used to control this excess.
A new and still experimental procedure uses boron-10, which
concentrates in the tumour. The patient is then irradiated with neutrons
which are strongly absorbed by the boron, to produce high-energy alpha
particles which kill the cancer.
For targeted alpha therapy (TAT), actinium-225 is readily available,
from which the daughter bismuth-213 can be obtained (via 3 alpha decays)
to label targeting molecules. The bismuth is obtained by elution from
an Ac-225/Bi-213 generator similar to the Mo-99/Tc-99 one. Bi-213 has a
46-minute half-life. The actinium-225 (half-life 10 days) is formed from
radioactive decay of radium-225, the decay product of long-lived
thorium-229, which is obtained from decay of uranium-233, which is
formed from Th-232 by neutron capture in a nuclear reactor.
Another radionuclide recovered from used nuclear fuel is lead-212,
with half-life of 10.6 hours, which can be attached to monoclonal
antibodies for cancer treatment. Its decay chain includes the
short-lived isotopes bismuth-212 by beta decay, polonium-212 by beta
decay and thallium-208 by alpha decay of the bismuth, with further alpha
and beta decays respectively to Pb-208, all over about an hour.
Considerable medical research is being conducted worldwide into the
use of radionuclides attached to highly specific biological chemicals
such as immunoglobulin molecules (monoclonal antibodies). The eventual
tagging of these cells with a therapeutic dose of radiation may lead to
the regression - or even cure - of some diseases.
Radioisotope Poisons
In 2006 Britain witnessed the apparent murder of one of its newer
citizens, a former Russian intelligence official, by poisoning with
radioactive polonium. His death was slow and excruciating.
Polonium has about 26 isotopes, all of which are radioactive.
Webelements periodic table says that it is 250 billion times more toxic
than hydrocyanic acid. It is readily soluble in weak acid. (It was the
first element discovered by Marie Curie, in 1898, and named after her
native Poland. Her daughter Irene was contaminated with polonium in a
laboratory accident and died of leukemia at the age of 59.)
Polonium-210 is the penultimate decay product of U-238, before it
alpha decays to become stable lead. It results from the beta decay of
Pb-210 (in the U-238 decay series) to Bi-210 which rapidly beta decays
to Po-210. This gives rise to its occurrence in nature, where uranium is
ubiquitous. However, because of its short (138 day) half life, very
little Po-210 would be found in uranium ore or mill tailings
(Webelements suggests 0.1 mg/tonne). Po-210 levels in soil would be even
less, but it is concentrated in tobacco and traces of it can be found
in smokers' urine.
Po-210 can also be made by neutron irradiation of Bi-209, and that is
most likely source of any significant quantity. Russia has used Po-210
as a heat source in short-life spacecraft and lunar rovers. It also
operates reactors using lead-bismuth cooling, which becomes contaminated
with Po-210 due to neutron bombardment.
Because its half-life is so short, a gram of Po-210 is about 5000
times as radioactive as a gram of radium - which sets the standard of
activity. But at 138 days its half life is long enough for it to be
manufactured, transported and administered before its loses its potency.
It would not put the carrier at much risk, since alpha radiation is
only really a hazard inside the body - a layer of skin is protection.
About 10 micrograms (2 GBq) was said to have been used, administered in a
cup of tea (it would be warm due to the decay).
However, simply dosing someone with polonium might not have much
effect if it simply went in one end and out the other in a day or two
without being absorbed from the gut. It would probably need to be
complexed on to an organic carrier which would enter the bloodstream and
take it to vital organs where it would stay. (This is what happens with
targeted alpha therapy (TAT) using very low levels of alpha-active
radioisotopes: the carriers take them to dispersed cancerous tissues
where they are needed.)
In Mr Litvinenko's case the intense alpha radiation was reportedly in
vital organs and sufficient to destroy them over three weeks. It was
apparently over one hundred times the dose used in TAT for cancer
treatment and the Po-210 is much longer-lived than isotopes used for
TAT. It could have been attached to something as simple as a sugar.
Supplies of radioisotopes
Most medical radioisotopes made in nuclear reactors are sourced from relatively few research reactors, including:
- NRU at Chalk River in Canada (supplied via MDS Nordion)
- HFR at Petten in Netherlands (supplied via IRE and Covidien)
- BR-2 at Mol in Belgium (supplied via IRE and Covidien)
- Maria in Poland (supplied via Covidien)
- Osiris & Orphee at Saclay in France (supplied via IRE)
- FRJ-2/ FRM-2 at Julich in Germany (supplied via IRE)
- LWR-15 at Rez in Czech Republic
- HFETR at Chengdu in China
- Safari in South Africa (supplied from NTP)
- Opal in Australia (supplied from ANSTO to domestic market, exports from 2016)
- ETRR-2 in Egypt (forthcoming: supplied to domestic market)
- Dimitrovgrad in Russia (Isotop-NIIAR)
Of fission radioisotopes, 40% of Mo-99 (for Tc-99m) comes from MDS
Nordion, 25% from Covidien (formerly Tyco), 17% from IRE and 10% from
NTP. For I-131, 75% is from IRE, 25% from NTP. Over 90% of the Mo-99
is made in five reactors: NRU in Canada (40%), HFR in Netherlands
(30%), BR-2 in Belgium (9%), Osiris in France (5%), and Safari-1 in
South Africa (10%). Canadian 2008 data gives 31% for NRU. Russia is
keen to increase its share of world supply, and in 2012 some 66% of its
radioisotope production was exported.
World demand for Mo-99 is 23,000 six-day TBq/yr.* It is mostly
prepared by fission of U-235 targets in a nuclear research reactor.
Most is produced using high-enriched uranium targets. The targets are
then processed to separate the Mo-99 and also to recover I-131. Opal,
Safari, and increasingly other reactors, are moving to low-enriched
uranium targets, which adds about 20% to production costs.
* 23,000 TBq is on basis of activity at 6
days from production reference point, ie 22% of nearly 100,000 TBq
required in production processing (given 66 hour half-life). This is
still about two days from the end of irradiation, so some 167,000 TBq/yr
must be made in the actual reactors to allow for cooling, processing
and decay en route to the users.
A number of incidents in 2008 pointed up shortcomings and unreliability in the supply of medical isotopes, particular technetium. As indicated above, most of the world's supply of Mo-99 for this comes from only five reactors, all of them 43 to 52 years old (in mid 2010). The Canadian and Netherlands reactors required major repairs over 2009-10 and were out of action for some time. Osiris is due to shut down in 2015. NRU at Chalk River has been re-licensed to 2016. A new 20 MW South Korean reactor at Busan is expected to be operating in 2016. An increasing supply shortfall of Tc-99 was forecast from 2010, and the IAEA is encouraging new producers. During the 2009-10 supply crisis, South Africa's (NECSA) Safari was able to supply 25% of the supply of Mo-99. Australia's Opal reactor has the capacity to produce half the world supply of it, and a much larger Mo production facility is planned to be on line in 2016 to meet one quarter of world demand. Also the processing and distribution of isotopes is complex and constrained, which can be critical when the isotopes concerned are short-lived. A need for increased production capacity and more reliable distribution is evident. The Mo-99 market is about $5 billion per year, according to NECSA.
A number of incidents in 2008 pointed up shortcomings and unreliability in the supply of medical isotopes, particular technetium. As indicated above, most of the world's supply of Mo-99 for this comes from only five reactors, all of them 43 to 52 years old (in mid 2010). The Canadian and Netherlands reactors required major repairs over 2009-10 and were out of action for some time. Osiris is due to shut down in 2015. NRU at Chalk River has been re-licensed to 2016. A new 20 MW South Korean reactor at Busan is expected to be operating in 2016. An increasing supply shortfall of Tc-99 was forecast from 2010, and the IAEA is encouraging new producers. During the 2009-10 supply crisis, South Africa's (NECSA) Safari was able to supply 25% of the supply of Mo-99. Australia's Opal reactor has the capacity to produce half the world supply of it, and a much larger Mo production facility is planned to be on line in 2016 to meet one quarter of world demand. Also the processing and distribution of isotopes is complex and constrained, which can be critical when the isotopes concerned are short-lived. A need for increased production capacity and more reliable distribution is evident. The Mo-99 market is about $5 billion per year, according to NECSA.
In 2009 the NEA set up the High-level Group
on the Security of Supply of Medical Radioisotopes (HLG-MR) to
strengthen the reliability of Mo-99 and Tc-99 supply in the short,
medium and long term. It reviewed the Mo-99 supply chain to identify the
key areas of vulnerability, the issues that need to be addressed and
the mechanisms that could be used to help resolve them. It requested an
economic study of the supply chain, and this was published in 2010 by
the NEA. The report identifies
possible changes needed. The historical development of the market has
an impact on the present economic situation, which is unsustainable. The
supply chain’s economic structure therefore needs to be changed to
attract additional investment in production capacity as well as the
necessary reserve capacity, and all supply chain participants worldwide
need to agree on and implement the changes needed.
The report predicts supply shortages from 2016, not simply from reactors but due to processing limitations. Historically reactor irradiation prices have been too low to attract new investment, and full cost recovery is needed to encourage new infrastructure. This will have little impact on end prices since irradiation only accounts for about 1% of product cost. Transport regulation and denial of shipment impede reliable supply. HEU use needs to be minimised, though conversion to LEU targets will reduce capacity. Outage reserve capacity needs to be sourced, valued and paid for by the supply chain. Fission is the most efficient and reliable means of production, but Canada and Japan are developing better accelerator-based techniques.
The report predicts supply shortages from 2016, not simply from reactors but due to processing limitations. Historically reactor irradiation prices have been too low to attract new investment, and full cost recovery is needed to encourage new infrastructure. This will have little impact on end prices since irradiation only accounts for about 1% of product cost. Transport regulation and denial of shipment impede reliable supply. HEU use needs to be minimised, though conversion to LEU targets will reduce capacity. Outage reserve capacity needs to be sourced, valued and paid for by the supply chain. Fission is the most efficient and reliable means of production, but Canada and Japan are developing better accelerator-based techniques.
The US Congress has called for all Mo-99 to be supplied by reactors
running on low-enriched uranium (LEU), instead of high-enriched uranium
(HEU). Also it has called for proposals for an LEU-based supply of Mo-99
for the US market. This supply should reach 111 six-day TBq per week by
mid-2013, a quarter of world demand. Tenders for this closed in June
2010.
In January 2009 Babcock & Wilcox (B&W) announced an agreement
with international isotope supplier Covidien to produce Mo-99
sufficient for half of US demand, if a new process involving an
innovative reactor and separation technology is successful. They plan
to use Aqueous Homogeneous Reactor (AHR) technology with low-enriched
uranium in small 100-200 kW units units where the fuel is mixed with the
moderator and the U-235 forms both the fuel and the irradiation
target.* A single production facility could have four such reactors.
B&W and Covidien expected a five-year lead time to first production.
B&W received $9 million towards this Medical Isotope Production
System (MIPS) in 2010 from the US government and completed the R&D
and conceptual design phase in 2012. However, in October 2012 Covidien
pulled out of the joint venture with B&W “after learning that the
time and cost involved with the project would be greater than originally
expected.” Covidien said that it was making significant long-term
capital investment in a new Tc-99m generator facility at our US plant,
and conversion from HEU- to LEU-based Mo-99 production at our processing
plant in the Netherlands.” At Russia's Kurchatov Institute the 20 kW
ARGUS AHR has operated since 1981, and R&D on producing Mo-99 from
it is ongoing.
* LEU is dissolved in acid then brought to
criticality in a 200-litre vessel. As fission proceeds the solution is
circulated through an extraction facility to remove the fission products
with Mo-99 and then back into the reactor vessel, which is at low
temperature and pressure.
Also in the USA, the University of Missouri was reported to be
planning a licence application to NRC to produce half of US requirements
of Mo-99 at its research reactor using low-enriched uranium targets,
but this did not proceed.
In Russia, the Research Institute of Atomic
Reactors (NIIAR or RIAR, with 3 reactors for isotope production) and
Trans-regional Izotop Association (VA Izotop JSC) established a joint
venture, Isotop-NIIAR to produce Mo-99 at Dimitrovgrad from 2010. Phase
1 of the Mo-99 production line with capacity of 1700 TBq/yr was
commissioned in December 2010, reaching capacity in May 2011, and Phase 2
started in February and commissioned in June 2012 will take capacity to
1480 TBq/yr (evidently 6-day activity). Earlier reports quoted 4800
TBq/yr, and Rosatom aims for 20% of the world Mo-99 market by 2014,
supplied internationally through Nordion. Since 2009, VA Izotop
has been authorized by Rosatom to control all isotope production and
radiological devices in Russia. In September 2010 JSC Izotop signed a
framework agreement with MDS Nordion to explore commercial opportunities
outside Russia on the basis of this Isotop-NIIAR JV, initially over ten
years.
Technetium can also be produced in small
quantities from cyclotrons and accelerators, in a cyclotron by
bombarding a Mo-100 target with a proton beam to produce Tc-99m
directly, or in a linear accelerator to generate Mo-99 by bombarding a
Mo-100 target with high-energy X-rays.
Main Mo-99 Production Reactors
| reactor | targets | capacity* | start | Est stop | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Belgium | BR-2 | HEU | 289 | 1961 | 2026 | ||||||
| Netherlands | HFR | HEU | 173 | 1961 | 2022 | ||||||
| Czech Rep | LVR-15 | HEU | 104 | 1989 | 2028 | ||||||
| Poland | Maria | HEU | 71 | 1974 | 2030 | ||||||
| Canada | NRU | HEU | 173 | 1957 | 2016 | ||||||
| Australia | OPAL | LEU | 37 | 2006 | 2030+ | ||||||
| France | OSIRIS | HEU | 44 | 1966 | 2015+ | ||||||
| Argentina | RA-3 | LEU | 15 | 1967 | 2027 | ||||||
| Russia | RIAR: three | HEU | 33 | 1961-70 | |||||||
| South Africa | Safari-1 | LEU | 111 | 1965 | 2025 | ||||||
| total | 1050 |
Planned Mo-99 Production Capacity
| reactor | targets | capacity* | start | |
|---|---|---|---|---|
| Russia | RIAR | LEU | 67-74 | 2013 |
| USA | B&W MIPS | LEU | 163 | 2015 |
| Germany | FRM-II | LEU | 72 | 2016 |
| Australia | OPAL | LEU | 133 | 2017 |
| China | CARR | LEU | 37 | 2017 |
| USA | Cocqui | LEU | 259 | 2017 |
* 6-day TBq/week
Source: Annex 1, 2 & 3, Supply of Medical Radioisotopes, March 2103, OECD/NEA
Cobalt-60 has mostly come from Candu power reactors by irradiation of
Co-59 in special rods, and production is being expanded. Production
sites include: Bruce B, Pickering and Gentilly in Canada; Embalse in
Argentina; Qinshan Phase III units 1 and 2 in China; Wolsong 1 and 2 in
South Korea (all Candu); and Leningrad 1 in Russia (RBMK). These were to
be joined by the Clinton and Hope Creek BWRs in USA from 2012.
Nuclear Medicine Wastes
The use of radioisotopes for medical diagnosis and treatments results in the generation of mainly Low-Level Waste
(LLW). This waste includes paper, rags, tools, clothing and filters,
which contain small amounts of mostly short-lived radioactivity. These
types of waste often undergo decay storage for periods of months to a
few years before being disposed of at urban land-fill sites.
When radiography sources have decayed to a point where they are no
longer emitting enough penetrating radiation for use in treatments, they
are considered as radioactive waste. Sources such as Co-60 are treated
as short-lived Intermediate-Level wastes (ILW). Other sources such as
Radium-226, used in cancer therapy, will however require long-term
storage and geological disposal as ILW, as a result of their higher
level of long-lived radioactivity.
Isotopes used in Medicine
Many radioisotopes are made in nuclear reactors, some in cyclotrons.
Generally neutron-rich ones and those resulting from nuclear fission
need to be made in reactors, neutron-depleted ones are made in
cyclotrons. There are about 40 activation product radioisotopes and
five fission product ones made in reactors.
Reactor Radioisotopes
(half-life indicated)
Bismuth-213 (46 min): Used for targeted alpha therapy (TAT), especially cancers, as it has a high energy (8.4 MeV).
Chromium-51 (28 d): Used to label red blood cells and quantify gastro-intestinal protein loss.
Cobalt-60 (5.27 yr): Formerly used for external beam radiotherapy, now used more for sterilising
Dysprosium-165 (2 h): Used as an aggregated hydroxide for synovectomy treatment of arthritis.
Erbium-169 (9.4 d): Use for relieving arthritis pain in synovial joints.
Holmium-166 (26 h): Being developed for diagnosis and treatment of liver tumours.
Iodine-125 (60 d): Used in cancer brachytherapy (prostate and brain),
also diagnostically to evaluate the filtration rate of kidneys and to
diagnose deep vein thrombosis in the leg. It is also widely used in
radioimmuno-assays to show the presence of hormones in tiny quantities.
Iodine-131 (8 d)*: Widely used in treating thyroid cancer and in
imaging the thyroid; also in diagnosis of abnormal liver function, renal
(kidney) blood flow and urinary tract obstruction. A strong gamma
emitter, but used for beta therapy.
Iridium-192 (74 d): Supplied in wire form for use as an internal
radiotherapy source for cancer treatment (used then removed). Beta
emitter.
Iron-59 (46 d): Used in studies of iron metabolism in the spleen.
Lead-212 (10.6 h): Used in TAT for cancers, with decay products Bi-212, Po-212, Tl-208.
Lutetium-177 (6.7 d): Lu-177 is increasingly important as it emits
just enough gamma for imaging while the beta radiation does the therapy
on small (eg endocrine) tumours. Its half-life is long enough to allow
sophisticated preparation for use. It is usually produced by neutron
activation of natural or enriched lutetium-176 targets.
Molybdenum-99 (66 h)*: Used as the 'parent' in a generator to produce technetium-99m.
Palladium-103 (17 d): Used to make brachytherapy permanent implant seeds for early stage prostate cancer.
Phosphorus-32 (14 d): Used in the treatment of polycythemia vera (excess red blood cells). Beta emitter.
Potassium-42 (12 h): Used for the determination of exchangeable potassium in coronary blood flow.
Rhenium-186 (3.8 d): Used for pain relief in bone cancer. Beta emitter with weak gamma for imaging.
Rhenium-188 (17 h): Used to beta irradiate coronary arteries from an angioplasty balloon.
Samarium-153 (47 h): Sm-153 is very effective in relieving the pain
of secondary cancers lodged in the bone, sold as Quadramet. Also very
effective for prostate and breast cancer. Beta emitter.
Selenium-75 (120 d): Used in the form of seleno-methionine to study the production of digestive enzymes.
Sodium-24 (15 h): For studies of electrolytes within the body.
Strontium-89 (50 d)*: Very effective in reducing the pain of prostate and bone cancer. Beta emitter.
Technetium-99m (6 h): Used in to image the skeleton and heart muscle
in particular, but also for brain, thyroid, lungs (perfusion and
ventilation), liver, spleen, kidney (structure and filtration rate),
gall bladder, bone marrow, salivary and lacrimal glands, heart blood
pool, infection and numerous specialised medical studies. Produced from
Mo-99 in a generator.
Xenon-133 (5 d)*: Used for pulmonary (lung) ventilation studies.
Ytterbium-169 (32 d): Used for cerebrospinal fluid studies in the brain.
Ytterbium-177 (1.9 h): Progenitor of Lu-177.
Yttrium-90 (64 h)*: Used for cancer brachytherapy and as silicate
colloid for the relieving the pain of arthritis in larger synovial
joints. Pure beta emitter and of growing significance in therapy.
Radioisotopes of caesium, gold and ruthenium are also used in brachytherapy.
* fission product
Cyclotron Radioisotopes
Carbon-11, Nitrogen-13, Oxygen-15, Fluorine-18: These are positron
emitters used in PET for studying brain physiology and pathology, in
particular for localising epileptic focus, and in dementia, psychiatry
and neuropharmacology studies. They also have a significant role in
cardiology. F-18 in FDG (fluorodeoxyglucose) has become very important
in detection of cancers and the monitoring of progress in their
treatment, using PET.
Cobalt-57 (272 d): Used as a marker to estimate organ size and for in-vitro diagnostic kits.
Copper-64 (13 h): Used to study genetic diseases affecting copper
metabolism, such as Wilson's and Menke's diseases, and for PET imaging
of tumours, and therapy.
Copper-67 (2.6 d): Beta emitter, used in therapy.
Fluorine-18 as FLT (fluorothymidine), F-miso (fluoromisonidazole), 18F-choline: tracer.
Gallium-67 (78 h): Used for tumour imaging and localisation of inflammatory lesions (infections).
Gallium-68 (68 min): Positron emitter used in PET and PET-CT units. Derived from germanium-68 in a generator.
Germanium-68 (271 d): Used as the 'parent' in a generator to produce Ga-68.
Indium-111 (2.8 d): Used for specialist diagnostic studies, eg brain studies, infection and colon transit studies.
Iodine-123 (13 h): Increasingly used for diagnosis of thyroid
function, it is a gamma emitter without the beta radiation of I-131.
Iodine-124: tracer.
Krypton-81m (13 sec) from Rubidium-81 (4.6 h): Kr-81m gas can yield
functional images of pulmonary ventilation, e.g. in asthmatic patients,
and for the early diagnosis of lung diseases and function.
Rubidium-82 (1.26 min): Convenient PET agent in myocardial perfusion imaging.
Strontium-82 (25 d): Used as the 'parent' in a generator to produce Rb-82.
Thallium-201 (73 h): Used for diagnosis of coronary artery disease
other heart conditions such as heart muscle death and for location of
low-grade lymphomas.
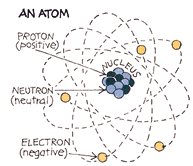
What are radioisotopes?
Many of the chemical elements have a number of isotopes. The isotopes
of an element have the same number of protons in their atoms (atomic
number) but different masses due to different numbers of neutrons. In an
atom in the neutral state, the number of external electrons also equals
the atomic number. These electrons determine the chemistry of the atom.
The atomic mass is the sum of the protons and neutrons. There are 82
stable elements and about 275 stable isotopes of these elements.
When a combination of neutrons and protons, which does not already
exist in nature, is produced artificially, the atom will be unstable and
is called a radioactive isotope or radioisotope. There are also a
number of unstable natural isotopes arising from the decay of primordial
uranium and thorium. Overall there are some 1800 radioisotopes.
At present there are up to 200 radioisotopes used on a regular basis, and most must be produced artificially.
Radioisotopes can be manufactured in several ways. The most common is
by neutron activation in a nuclear reactor. This involves the capture
of a neutron by the nucleus of an atom resulting in an excess of
neutrons (neutron rich). Some radioisotopes are manufactured in a
cyclotron in which protons are introduced to the nucleus resulting in a
deficiency of neutrons (proton rich).
The nucleus of a radioisotope usually becomes stable by emitting an
alpha and/or beta particle (or positron). These particles may be
accompanied by the emission of energy in the form of electromagnetic
radiation known as gamma rays. This process is known as radioactive
decay.
Radioactive products which are used in medicine are referred to as radiopharmaceuticals.
Radiation and Life
December 2012
http://www.world-nuclear.org/info/Safety-and-Security/Radiation-and-Health/Radiation-and-Life/#.UhbNhn-N6So
http://www.world-nuclear.org/info/Safety-and-Security/Radiation-and-Health/Radiation-and-Life/#.UhbNhn-N6So
"Life on earth has developed with an ever present background of
radiation. It is not something new, invented by the wit of man:
radiation has always been there."
Eric J Hall, Professor of Radiology, College of Physicians and
Surgeons, Columbia University, New York, in his book "Radiation and
Life".
Radiation is energy travelling through space.
Sunshine is one of the most familiar forms of radiation. It
delivers light, heat and suntans. We limit its effect on us with
sunglasses, shade, hats, clothes and sunscreen.
There would be no life on Earth without lots of sunlight, but we
have increasingly recognised that too much of it on our persons is
not a good thing. In fact it may be dangerous, so we control our
exposure to it.
Sunshine consists of radiation in a range of wavelengths from
long-wave infra-red to short-wavelength ultraviolet, which creates
the hazard.
Beyond ultraviolet are higher energy kinds of radiation which
are used in medicine and which we all get in low doses from space,
from the air, and from the earth and rocks. Collectively we can
refer to these kinds of radiation as ionising
radiation. It can cause damage to matter, particularly
living tissue. At high levels it is therefore dangerous, so it is
necessary to control our exposure.

Living things have evolved in an environment which has
significant levels of ionising radiation. Furthermore, many of us
owe our lives and health to such radiation produced artificially.
Medical and dental X-rays discern hidden problems. Other kinds of
ionising radiation are used to diagnose ailments, and some people
are treated with radiation to cure disease. We all benefit from a
multitude of products and services made possible by the careful use
of such radiation.
Background radiation is that which is naturally and inevitably
present in our environment. Levels of this can vary greatly. People
living in granite areas or on mineralised sands receive more
terrestrial radiation than others, while people living or working
at high altitudes receive more cosmic radiation. A lot of our
natural exposure is due to radon, a gas which seeps from the
Earth's crust and is present in the air we breathe.
Unstable Atoms
Ionising radiation comes from the nuclei of atoms, the basic
building blocks of matter.
Each element exists in the form of atoms with several different
sized nuclei, called isotopes.
Most atoms are stable; a carbon-12 atom for example remains a
carbon-12 atom forever, and an oxygen-16 atom remains an oxygen-16
atom forever. But certain atoms change or disintegrate into
totally new atoms. These kinds of atoms are said to be 'unstable'
or 'radioactive'. An unstable atom has excess internal energy, with
the result that the nucleus can undergo a spontaneous change
towards a more stable form. This is called 'radioactive decay'.
Unstable isotopes (which are thus radioactive) are called
radioisotopes. Some elements, eg uranium, have no stable
isotopes.
Atomic Decay
When an atom of a radioisotope decays, it gives off some of its
excess energy as radiation in the form of gamma rays or fast-moving
sub-atomic particles. If it decays with emission of an alpha or
beta particle, it becomes a new element. One can describe the
emissions as gamma, beta and alpha radiation. All the time, the
atom is progressing in one or more steps towards a stable state
where it is no longer radioactive.
Another source of nuclear radioactivity is when one form of a
radioisotope changes into another form, or isomer, releasing a
gamma ray in the process. The excited form is signified with an "m"
(meta) beside its atomic number, eg technetium-99m (Tc-99m) decays
to Tc-99. Gamma rays are often emitted with alpha or beta radiation
also, as the nucleus decays to a less excited state.
Apart from the normal measures of mass and volume, the amount of
radioactive material is given
in becquerel (Bq), a measure which
enables us to compare the typical radioactivity of some natural and
other materials. A becquerel is one atomic decay per second*, and
each disintegration produces some ionising radiation.
*A former unit of (radio)activity is the Curie - 1 Bq is 27 x
10-12 curies.
Radioactivity in some natural
and other materials
| 1 adult human (100 Bq/kg) | 7000 Bq |
| 1 kg of coffee | 1000 Bq |
| 1 kg superphosphate fertiliser | 5000 Bq |
| The air in a 100 sq metre Australian home (radon) | 3000 Bq |
| The air in many 100 sq metre European homes (radon) | up to 30 000 Bq |
| 1 household smoke detector (with americium) | 30 000 Bq |
| Radioisotope for medical diagnosis | 70 million Bq |
| Radioisotope source for medical therapy | 100 000 000 million Bq (100 TBq) |
| 1 kg 50-year old vitrified high-level nuclear waste | 10 000 000 million Bq (10 TBq) |
| 1 luminous Exit sign (1970s) | 1 000 000 million Bq (1 TBq) |
| 1 kg uranium | 25 million Bq |
| 1 kg uranium ore (Canadian, 15%) | 26 million Bq |
| 1 kg uranium ore (Australian, 0.3% | 500 000 Bq |
| 1 kg low level radioactive waste | 1 million Bq |
| 1 kg of coal ash | 2000 Bq |
| 1 kg of granite | 1000 Bq |
N.B. Though the intrinsic radioactivity
is the same, the radiation dose received by someone handling a
kilogram of high-grade uranium ore will be much greater than for
the same exposure to a kilogram of separated uranium, since the ore
contains a number of short-lived decay products (see section on
Radioactive Decay), while the uranium haas a very long
half-life.
Half-life
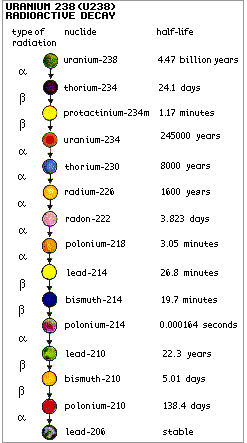
Atoms in a radioactive substance decay
in a random fashion but at a characteristic rate. The length of
time this takes, the number of steps required and the kinds of
radiation released at each step are well known.
The half-life is the time taken for
half of the atoms of a radioactive substance to decay. Half-lives
can range from less than a millionth of a second to millions of
years depending on the element concerned. After one half-life the
level of radioactivity of a substance is halved, after two
half-lives it is reduced to one quarter, after three half-lives to
one-eighth, and so on.
All uranium atoms are mildly
radioactive and decay through a number of steps on the way to
becoming stable lead. Each step has a different half life, and a
characteristic type of radiation. The shorter-lived each kind of
radioisotope in the decay series, the more radiation it emits per
unit mass. Much of the natural radioactivity in rocks and soil
comes from the uranium-238 (U-238) decay chain (but not from the
uranium itself).

Types of Ionising Radiation
Here we are concerned mainly with
ionising radiation from the atomic nucleus. It occurs in two forms,
rays and particles, at the high frequency end of the energy
spectrum.
Ionising radiation produces
electrically-charged particles called ions in the materials it
strikes. This process is called ionisation. In the large chemical
molecules of which all living things are made, the changes caused
may be biologically important.
There are several types of ionising radiation:
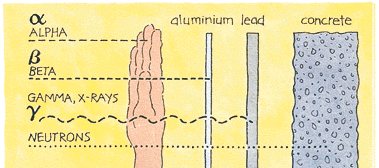
X-rays and gamma rays,
like light, represent energy transmitted in a wave without the
movement of material, just as heat and light from a fire or the sun
travels through space. X-rays and gamma rays are virtually
identical except that X-rays are generally produced artificially
rather than coming from the atomic nucleus. But unlike light,
X-rays and gamma rays have great penetrating power and can pass
through the human body. Mass in the form of concrete, lead or water
are used to shield us from them.
Alpha
particles consist of two protons and two neutrons, in
the form of atomic nuclei. They thus have a positive electrical
charge and are emitted from naturally-occurring heavy elements such
as uranium and radium, as well as from some man-made elements.
Because of their relatively large size, alpha particles collide
readily with matter and lose their energy quickly. They therefore
have little penetrating power and can be stopped by the first layer
of skin or a sheet of paper.
However, if alpha sources are taken
into the body, for example by breathing or swallowing radioactive
dust, alpha particles can affect the body's cells. Inside the body,
because they give up their energy over a relatively short distance,
alpha particles can inflict more severe biological damage than
other types of radiation.
Beta
particles are fast-moving electrons ejected from the
nuclei of many kinds of radioactive atoms. These particles are much
smaller than alpha particles and can penetrate up to 1 to 2
centimetres of water or human flesh. They can be stopped by a
sheet of aluminium a few millimetres thick.
Cosmic
radiation consists of very energetic particles,
mostly protons, which bombard the Earth from outer space. It is
more intense at higher altitudes than at sea level where the
Earth's atmosphere is most dense and gives the greatest
protection.
Neutrons are
particles which are also very penetrating. On Earth they mostly
come from the splitting, or fissioning, of certain atoms inside a
nuclear reactor. Water and concrete are the most commonly used
shields against neutron radiation from the core of the nuclear
reactor.
It is important to understand that
alpha, beta, gamma and X-radiation does not cause the body to
become radioactive. However, most materials in their natural state
(including body tissue) contain measurable amounts of
radioactivity.
Measuring Ionising Radiation
Grays and
Sieverts
The human senses cannot detect
radiation or discern whether a material is radioactive. However, a
variety of instruments can detect and measure radiation reliably
and accurately.
The amount of ionising radiation, or
'dose', received by a person is measured in terms of the energy
absorbed in the body tissue, and is expressed
in gray. One gray (Gy) is one joule deposited
per kilogram of mass.
Equal exposure to different types of
radiation expressed as gray do not however necessarily produce
equal biological effects. One gray of alpha radiation, for example,
will have a greater effect than one gray of beta radiation. When we
talk about radiation effects, we therefore express the radiation as
effective dose, in a unit called
the sievert (Sv).
Regardless of the type of radiation,
one sievert (Sv) of radiation produces the same biological
effect.
Smaller quantities are expressed in
'millisievert' (one thousandth) or 'microsievert' (one millionth)
of a sievert. We will use the most common
unit, millisievert (mSv), here.
What are the health risks from ionising radiation?
It has been known for many years that
large doses of ionising radiation, very much larger than background
levels, can cause a measurable increase in cancers and leukemias
('cancer of the blood') after some years delay. It must also be
assumed, because of experiments on plants and animals, that
ionising radiation can also cause genetic mutations that affect
future generations, although there has been no evidence of
radiation-induced mutation in humans. At very high levels,
radiation can cause sickness and death within weeks of exposure -
see Table.
The degree of damage caused by
radiation depends on many factors - dose, dose rate, type of
radiation, the part of the body exposed, age and health, for
example. Embryos including the human fetus are particularly
sensitive to radiation damage.
But what are the chances of developing
cancer from low doses of radiation? The prevailing assumption is
that any dose of radiation, no matter how small, involves a
possibility of risk to human health. However there is no scientific
evidence of risk at doses below about 50 millisievert in a short
time or about 100 millisievert per year (40 times average annual
dose from natural background). At lower doses and dose rates, up to
at least 10 millisieverts per year (4 times average background),
the evidence suggests that beneficial effects are as likely as
adverse ones.
Higher accumulated doses of radiation
might produce a cancer which would only be observed several - up to
twenty - years after the radiation exposure. This delay makes it
impossible to say with any certainty which of many possible agents
were the cause of a particular cancer. In western countries, about
a quarter of people die from cancers, with smoking, dietary
factors, genetic factors and strong sunlight being among the main
causes. Radiation is a weak carcinogen, but undue exposure could
certainly increase health risks.
The body has defence mechanisms against
damage induced by radiation as well as by chemical and other
carcinogens. These can be stimulated by low levels of exposure, or
overwhelmed by very high levels.
On the other hand, large doses of
radiation directed specifically at a tumour are used in radiation
therapy to kill cancerous cells, and thereby often save lives
(usually in conjunction with chemotherapy or surgery). Much larger
doses are used to kill harmful bacteria in food, and to sterilise
bandages and other medical equipment. Radiation has become a
valuable tool in our modern world. See also The Peaceful
Atom in this series.
Tens of thousands of people in each
technically advanced country work in medical and industrial
environments where they may be exposed to radiation above
background levels. Accordingly they wear monitoring 'badges' while
at work, and their exposure is carefully monitored. The health
records of these occupationally exposed groups often show that they
have lower rates of mortality from cancer and other causes than the
general public and, in some cases, significantly lower rates than
other workers who do similar work without being exposed to
radiation.
Radiation levels and their
effects
The following table gives an indication
of the likely effects of a range of whole-body radiation doses and
dose rates to individuals:
| 10,000 mSv (10
sieverts) as a short-term and whole-body dose would cause immediate
illness, such as nausea and decreased white blood cell count, and
subsequent death within a few weeks. Between 2 and 10 sieverts in a short-term dose would cause severe radiation sickness with increasing likelihood that this would be fatal. |
| 1,000 mSv (1 sievert) in a short-term
dose is about the threshold for causing immediate radiation
sickness in a person of average physical attributes, but would be
unlikely to cause death. Above 1000 mSv, severity of illness
increases with dose. If doses greater than 1000 mSv occur over a long period they are unlikely to have health effects, but they may create some risk that cancer will develop many years later. |
| 250 mSv as short-term dose was maximum
allowable for workers controlling the Fukushima
accident. |
| Above about 100 mSv, the probability of cancer (rather than the severity of
illness) increases with dose. The estimated risk of fatal cancer is 5 of every 100 persons exposed to a dose of 1000 mSv (ie. if the normal incidence of fatal cancer were 25%, a 1000 mSv dose would increase it to 30%). |
| 50 mSv is, conservatively, the lowest
dose at which there is any evidence of cancer being caused in
adults. It is also the highest dose which is allowed by regulation
in any one year of occupational exposure. Dose rates greater than
50 mSv/yr arise from natural background levels in several parts of
the world but do not cause any discernible harm to local
populations.
|
| 20 mSv/yr averaged over
5 years is the limit for radiological personnel such as employees
in the nuclear industry, uranium or mineral sands miners and
hospital workers (who are all closely monitored).
|
| 10 mSv/yr is the maximum actual dose rate
received by any Australian uranium miner.
|
| 3-5 mSv/yr is the
typical dose rate (above background) received by uranium miners in
Australia and Canada.
|
| 3 mSv/yr (approx) is the typical
background radiation from natural sources in North America,
including an average of almost 2 mSv/yr from radon in air.
|
| 2.5 mSv/yr (approx) is
the typical background radiation from natural sources, including an
average of 0.7 mSv/yr from radon in air. The minimum dose received
by all humans anywhere on Earth is about 1.5 mSv/yr.
|
| 0.3-0.6 mSv/yr is a typical range of dose
rates from artificial sources of radiation, mostly medical.
|
| 0.05 mSv/yr, a very small
fraction of natural background radiation, is the design target for
maximum radiation at the perimeter fence of a nuclear electricity
generating station. In practice the actual dose is less.
|
Background Radiation
Naturally-occurring background
radiation is the main source of exposure for most people. Levels
typically range from about 1.5 to 3.5 millisievert per year but can
be more than 50 mSv/yr. The highest known level of background
radiation affecting a substantial population is in Kerala and
Madras States in India where some 140,000 people receive doses
which average over 15 millisievert per year from gamma radiation,
in addition to a similar dose from radon. Comparable levels occur
in Brazil and Sudan, with average exposures up to about 40 mSv/yr
to many people.
Several places are known in Iran, India
and Europe where natural background radiation gives an annual dose
of more than 50 mSv and up to 260 mSv (at Ramsar in Iran). Lifetime
doses from natural radiation range up to several thousand
millisievert. However, there is no evidence of increased cancers or
other health problems arising from these high natural levels.
Ionising
Radiation
The Earth is radioactive, due to
the decay of natural long-lived radioisotopes. Radioactive decay
results in the release of ionizing radiation. As well as the
Earth's radioactivity we are naturally subject to cosmic radiation
from space. In addition to both these, we collect some radiation
doses from artificial sources such as X-rays. We may also collect
an increased cosmic radiation dose due to participating in high
altitude activities such as flying or skiing. The average adult
contains about 13 mg of radioactive potassium-40 in body tissue -
we therefore even irradiate one another at close quarters! The
relative importance of these various sources is indicated:
| Typical µSv/yr |
Range | |
| Natural: | ||
| Terrestrial + house: radon | 200 | 200-100,000 |
| Terrestrial + house: gamma | 600 | 100-1000 |
| Cosmic (at sea level) | 300 | |
+20 for every 100m elevation
|
0-500 | |
| Food, drink & body tissue | 400 | 100-1000 |
Total
|
1500 (plus altitude adjustment) | |
| Artificial: | ||
| From nuclear weapons tests | 3 | |
| Medical (X-ray, CT etc. average) | 370 | up to 75,000 |
| From nuclear energy | 0.3 | |
| From coal burning | 0.1 | |
| From household appliances | 0.4 | |
Total
|
375 | |
| Behavioural: | ||
| Skiing holiday | 8 per week | |
| Air travel in jet airliner | 1.5-5 per hour | up to 5000/yr |
The International Commission for Radiological Protection
recommends, in addition to background, the following exposure
limits:
for general public, 1,000 (i.e. 1 mSv/yr)
for nuclear worker 20,000 (i.e. 20 mSv/yr) averaged over 5
consecutive years
|
||
Sources: Australian Radiation Protection & Nuclear Safety Agency, Health & Safety Executive (UK), Australian Nuclear Science & Technology Organization, various
Man-made Radiation
Ionising radiation is also generated in
a range of medical, commercial and industrial activities. The most
familiar and, in national terms, the largest of these sources of
exposure is medical X-rays. A typical breakdown between natural
background and artificial sources of radiation is shown in the pie
chart.
Natural radiation contributes about 88%
of the annual dose to the population, and medical procedures most
of the remaining 12%. Natural and artificial radiations are not
different in kind or effect.
Protection from Radiation
Because exposure to high levels of
ionising radiation carries a risk, should we attempt to avoid it
entirely? Even if we wanted to, this would be impossible. Radiation
has always been present in the environment and in our bodies.
However, we can and should minimise unnecessary exposure to
significant levels of man-made radiation.
Radiation is very easily detected.
There is a range of simple, sensitive instruments capable of
detecting minute amounts of radiation from natural and
anthropogenic sources.
There are four ways in which people are
protected from identified radiation sources:
Limiting
Time: For people who are exposed to radiation in
addition to natural background radiation through their work, the
dose is reduced and the risk of illness essentially eliminated by
limiting exposure time.
Distance: In the
same way that heat from a fire is less the further away you are,
the intensity of radiation decreases with distance from its
source.
Shielding: Barriers of lead, concrete or
water give good protection from penetrating radiation such as gamma
rays. Radioactive materials are therefore often stored or handled
under water, or by remote control in rooms constructed of thick
concrete or even lined with lead.
Containment: Radioactive materials are
confined and kept out of the environment. Radioactive isotopes for
medical use, for example, are dispensed in closed handling
facilities, while nuclear reactors operate within closed systems
with multiple barriers which keep the radioactive materials
contained. Rooms have a reduced air pressure so that any leaks
occur into the room and not out from the room.
Standards and Regulations
Radiation protection standards are
based on the conservative assumption that the risk is directly
proportional to the dose, even at the lowest levels, though there
is no evidence of risk at low levels. This assumption, called the
'linear no-threshold (LNT) hypothesis', is recommended for
radiation protection purposes only such as setting allowable levels
of radiation exposure of individuals. It cannot properly be used
for predicting the consequences of an actual exposure to low levels
of radiation. For example, it suggests that, if the dose is halved
from a high level where effects have been observed, there will be
half the effect, and so on. This could be very misleading if
applied to a large group of people exposed to trivial levels of
radiation and could lead to inappropriate actions to avert the
doses.
Much of the evidence which has led to
today's standards derives from the atomic bomb survivors in 1945,
who were exposed to high doses incurred in a very short time. In
setting occupational risk estimates, some allowance has been made
for the body's ability to repair damage from small exposures, but
for low-level radiation exposure the degree of protection may be
unduly conservative.
In any country, radiation protection
standards are set by government authorities, generally in line with
recommendations by the International Commission on Radiological
Protection (ICRP),
and coupled with the requirement to keep exposure as low as
reasonably achievable (ALARA) - taking into account social and
economic factors. The authority of the ICRP comes from the
scientific standing of its members and the merit of its
recommendations.
The three key points of the ICRP's
recommendations are:
- Justification. No practice should be adopted unless its
introduction produces a positive net benefit.
- Optimisation. All exposures should be kept as low as reasonably
achievable, economic and social factors being taken into
account.
- Limitation. The exposure of individuals should not exceed the limits recommended for the appropriate circumstances.
The ICRP recommends that the maximum permissible dose for occupational exposure should be 20 millisievert per year averaged over five years (ie 100 millisievert in 5 years, about 8 time average dose from natural background) with a maximum of 50 millisievert in any one year. For public exposure, 1 millisievert per year averaged over five years is the limit. In both categories, the figures are over and above background levels, and exclude medical exposure.
Understanding Radiation
Ionising radiation has been studied very intensively for more than a century. Compared with many things which influence human health, it is well understood scientifically. The main internationally-recognised authority on ionising radiation is the UN Scientific Committee on the Effects of Atomic Radiation (UNSCEAR), set up in 1955. Its mandate is to assess and report levels and effects of exposure to ionizing radiation.Public, and even medical practitioners', understanding of ionising radiation is generally low, which gives scope for generating misinformation on the subject resulting in fear. This is compounded by the invisible nature of radiation, and a frequent confusion of units used to describe both radioactivity and radiation exposure. However, radiation is easily detectable and precisely measurable. Furthermore, the effects are well-known, though contested from non-scientific sources and popular folklore.
Lack of understanding and properly measuring radiation has major public health effects. In 1987 in Brazil an old radiotherapy source the size of a small cup stolen from an abandoned hospital caused four deaths, 20 cases of radiation sickness and significant contamination of many more. In 1986 the Chernobyl nuclear accident caused a few (preventable) deaths from thyroid cancer and massive psycho-social impact due to relocation of over 100,000 people, mostly unnecessarily. (It also caused 28 deaths among clean-up workers who received high radiation exposure.) For members of the public, fear of radiation was much more devastating than radiation itself.
The body has defence mechanisms against damage induced by radiation as well as by chemical carcinogens. These can be stimulated by low levels of exposure, or overwhelmed by very high levels.*
* Tens of thousands of people in each technically-advanced country work in medical and industrial environments where they may be exposed to radiation above background levels. Accordingly they wear monitoring "badges" while at work, and their exposure is carefully monitored. The health records of these occupationally exposed groups often show that they have lower rates of mortality from cancer and other causes than the general public and, in some cases, significantly lower rates than other workers who do similar work without being exposed to radiation.
The occurrence of cancer is not uniform across the world population, and because of local differences it is not easy to see whether or not there is any association between low occupational radiation doses and excess cancers. This question has been studied closely in a number of areas and work continues, but so far no conclusive evidence has emerged to indicate that cancers are more frequent in radiation workers (or those living near nuclear facilities) than in other people of similar ages in western countries.
Further information on the subject can be found in the information paper on Nuclear Radiation and Health Effects.
The ARPANSA web site section on Radiation and Health is also valuable.
Hi,
BalasHapusHealthline.com recently launched a free interactive "Human Body Maps" tool. I thought your readers would be interested in our body map of the kidney: http://www.healthline.com/human-body-maps/kidney
It would be much appreciated if you could include this tool on http://zadandunia.blogspot.com/2013/08/nuclear-medicine-uses-radiation-to.html and / or share with friends and followers. Please let me know if you have any questions.
Thank you in advance.
Warm Regards,
Maggie Danhakl- Assistant Marketing Manager
p: 415-281-3124 f: 415-281-3199
Healthline Networks, Inc. * Connect to Better Health
660 Third Street, San Francisco, CA 94107 www.healthline.com