Radioactive waste
From Wikipedia, the free encyclopedia
Radioactive wastes are wastes that contain radioactive material. Radioactive wastes are usually by-products of nuclear power generation and other applications of nuclear fission or nuclear technology, such as research and medicine. Radioactive waste is hazardous to most forms of life and the environment, and is regulated by government agencies in order to protect human health and the environment.
Radioactivity naturally decays over time,
so radioactive waste has to be isolated and confined in appropriate
disposal facilities for a sufficient period of time until it no longer
poses a hazard. The period of time waste must be stored depends on the
type of waste and radioactive isotopes. It can range from a few days for
very short-lived isotopes to millions of years for spent nuclear fuel.
Current major approaches to managing radioactive waste have been
segregation and storage for short-lived waste, near-surface disposal for
low and some intermediate level waste, and deep burial or partioning / transmutation for the high-level waste.
A summary of the amounts of radioactive waste and management
approaches for most developed countries are presented and reviewed
periodically as part of the International Atomic Energy Agency (IAEA) Joint Convention on the Safety of Spent Fuel Management and on the Safety of Radioactive Waste Management.[1]
Contents
Nature and significance of radioactive waste
Radioactive waste typically comprises a number of radioisotopes: unstable configurations of elements that decay, emitting ionizing radiation
which can be harmful to humans and the environment. Those isotopes emit
different types and levels of radiation, which last for different
periods of time.
Physics
Main article: fission product yield
| Prop: Unit: |
t½ a |
Yield % |
Q * keV |
βγ * |
|---|---|---|---|---|
| 155Eu | 4.76 | .0803 | 252 | βγ |
| 85Kr | 10.76 | .2180 | 687 | βγ |
| 113mCd | 14.1 | .0008 | 316 | β |
| 90Sr | 28.9 | 4.505 | 2826 | β |
| 137Cs | 30.23 | 6.337 | 1176 | βγ |
| 121mSn | 43.9 | .00005 | 390 | βγ |
| 151Sm | 96.6 | .5314 | 77 | β |
| Prop: Unit: |
t½ Ma |
Yield % |
Q * KeV |
βγ * |
|---|---|---|---|---|
| 99Tc | 0.211 | 6.1385 | 294 | β |
| 126Sn | 0.230 | 0.1084 | 4050 | βγ |
| 79Se | 0.327 | 0.0447 | 151 | β |
| 93Zr | 1.53 | 5.4575 | 91 | βγ |
| 135Cs | 2.3 | 6.9110 | 269 | β |
| 107Pd | 6.5 | 1.2499 | 33 | β |
| 129I | 15.7 | 0.8410 | 194 | βγ |
| Hover underlined: more info | ||||
The radioactivity of all nuclear waste diminishes with time. All radioisotopes contained in the waste have a half-life—the time it takes for any radionuclide to lose half of its radioactivity—and eventually all radioactive waste decays into non-radioactive elements (i.e., stable isotopes). Certain radioactive elements (such as plutonium-239) in “spent” fuel will remain hazardous to humans and other creatures for hundreds or thousands of years. Other radioisotopes
remain radioactive for millions of years (though most of these products
have so little activity as a result of their long half-lives that their
radiation is lost in the background level). Thus, these wastes must be
shielded for centuries and isolated from the living environment for millennia.[2]
Since radioactive decay follows the half-life rule, the rate of decay
is inversely proportional to the duration of decay. In other words, the
radiation from a long-lived isotope like iodine-129 will be much less intense than that of a short-lived isotope like iodine-131.[3] The two tables show some of the major radioisotopes, their half-lives, and their radiation yield as a proportion of the yield of fission of uranium-235.
The energy and the type of the ionizing radiation emitted by a radioactive substance are also important factors in determining its threat to humans.[4] The chemical properties of the radioactive element will determine how mobile the substance is and how likely it is to spread into the environment and contaminate humans.[5]
This is further complicated by the fact that many radioisotopes do not
decay immediately to a stable state but rather to radioactive decay products within a decay chain before ultimately reaching a stable state.
Pharmacokinetics
|
Actinides and fission products by half-life
|
||||||||
|---|---|---|---|---|---|---|---|---|
| Actinides[6] by decay chain | Half-life range (a) |
Fission products by yield[7] | ||||||
| 4n | 4n+1 | 4n+2 | 4n+3 | |||||
| 4.5–7% | 0.04–1.25% | <0.001% | ||||||
| 228Ra№ | 4 – 6 | † | 155Euþ | |||||
| 244Cm | 241Puƒ | 250Cf | 227Ac№ | 10 – 29 | 90Sr | 85Kr | 113mCdþ | |
| 232Uƒ | 238Pu | 243Cmƒ | 29 – 97 | 137Cs | 151Smþ | 121mSn | ||
| 249Cfƒ | 242mAmƒ | 141 – 351 |
No fission products have a half-life in the range of 100 – 210k years… |
|||||
| 241Am | 251Cfƒ[8] | 430 – 900 | ||||||
| 226Ra№ | 247Bk | 1.3k – 1.6k | ||||||
| 240Pu | 229Th | 246Cm | 243Am | 4.7k – 7.4k | ||||
| 245Cmƒ | 250Cm | 8.3k – 8.5k | ||||||
| 239Puƒ | 24.1k | |||||||
| 230Th№ | 231Pa№ | 32k – 76k | ||||||
| 236Npƒ | 233Uƒ | 234U№ | 150k – 250k | ‡ | 99Tc₡ | 126Sn | ||
| 248Cm | 242Pu | 327k – 375k | 79Se₡ | |||||
| 1.53M | 93Zr | |||||||
| 237Np | 2.1M – 6.5M | 135Cs₡ | 107Pd | |||||
| 236U | 247Cmƒ | 15M – 24M | 129I₡ | |||||
| 244Pu№ | 80M |
...nor beyond 15.7M[9] |
||||||
| 232Th№ | 238U№ | 235Uƒ№ | 0.7G – 14G | |||||
|
Legend for superscript symbols ₡ has thermal neutron capture cross section in the range of 8 – 50 barns ƒ fissile m metastable isomer № naturally occurring radioactive material (NORM) þ neutron poison (thermal neutron capture cross section greater than 3k barns) † range 4a – 97a: Medium-lived fission product ‡ over 200ka: Long-lived fission product |
||||||||
Exposure to high levels of radioactive waste may cause serious harm or death. Treatment of an adult animal with radiation or some other mutation-causing effect, such as a cytotoxic anti-cancer drug, may cause cancer in the animal. In humans it has been calculated that a 5 sievert dose is usually fatal, and the lifetime risk of dying from radiation-induced cancer from a single dose of 0.1 sieverts is 0.8%, increasing by the same amount for each additional 0.1 sievert increment of dosage.[10] Ionizing radiation causes deletions in chromosomes.[11] If a developing organism such as an unborn child is irradiated, it is possible a birth defect may be induced, but it is unlikely this defect will be in a gamete or a gamete-forming cell. The incidence of radiation-induced mutations in humans is undetermined, due to flaws in studies done to date.[12]
Depending on the decay mode and the pharmacokinetics of an element (how the body processes it and how quickly), the threat due to exposure to a given activity of a radioisotope will differ. For instance iodine-131 is a short-lived beta and gamma emitter, but because it concentrates in the thyroid gland, it is more able to cause injury than caesium-137 which, being water soluble, is rapidly excreted in urine. In a similar way, the alpha emitting actinides and radium are considered very harmful as they tend to have long biological half-lives and their radiation has a high relative biological effectiveness,
making it far more damaging to tissues per amount of energy deposited.
Because of such differences, the rules determining biological injury
differ widely according to the radioisotope, and sometimes also the
nature of the chemical compound which contains the radioisotope.
Sources of waste
Radioactive waste comes from a number of sources. The majority of
waste originates from the nuclear fuel cycle and nuclear weapons
reprocessing.[citation needed]
Other sources include medical and industrial wastes, as well as
naturally occurring radioactive materials (NORM) that can be
concentrated as a result of the processing or consumption of coal, oil
and gas, and some minerals, as discussed below.
Nuclear fuel cycle
Main articles: Nuclear fuel cycle and Spent nuclear fuel
- This article is about radioactive waste, for contextual information, see Nuclear power.
Front end
Waste from the front end of the nuclear fuel cycle is usually alpha-emitting waste from the extraction of uranium. It often contains radium and its decay products.
Uranium dioxide (UO2) concentrate from mining is not very radioactive - only a thousand or so times as radioactive as the granite used in buildings. It is refined from yellowcake (U3O8), then converted to uranium hexafluoride gas (UF6). As a gas, it undergoes enrichment to increase the U-235 content from 0.7% to about 4.4% (LEU). It is then turned into a hard ceramic oxide (UO2) for assembly as reactor fuel elements.[13]
The main by-product of enrichment is depleted uranium (DU), principally the U-238 isotope, with a U-235 content of ~0.3%. It is stored, either as UF6 or as U3O8. Some is used in applications where its extremely high density makes it valuable such as anti-tank shells, even sailboat keels on at least one occasion.[14] It is also used with plutonium for making mixed oxide fuel (MOX) and to dilute, or downblend, highly enriched uranium from weapons stockpiles which is now being redirected to become reactor fuel.
Back end
See also: Nuclear reprocessing
The back end of the nuclear fuel cycle, mostly spent fuel rods, contains fission products that emit beta and gamma radiation, and actinides that emit alpha particles, such as uranium-234, neptunium-237, plutonium-238 and americium-241, and even sometimes some neutron emitters such as californium (Cf). These isotopes are formed in nuclear reactors.
It is important to distinguish the processing of uranium to make fuel from the reprocessing
of used fuel. Used fuel contains the highly radioactive products of
fission (see high level waste below). Many of these are neutron
absorbers, called neutron poisons
in this context. These eventually build up to a level where they absorb
so many neutrons that the chain reaction stops, even with the control
rods completely removed. At that point the fuel has to be replaced in
the reactor with fresh fuel, even though there is still a substantial
quantity of uranium-235 and plutonium
present. In the United States, this used fuel is stored, while in
countries such as Russia, the United Kingdom, France, Japan and India,
the fuel is reprocessed to remove the fission products, and the fuel can
then be re-used. This reprocessing involves handling highly radioactive
materials, and the fission products removed from the fuel are a
concentrated form of high-level waste as are the chemicals used in the
process. While these countries reprocess the fuel carrying out single
plutonium cycles, India is the only country known to be planning
multiple plutonium recycling schemes.[15]
Fuel composition and long term radioactivity
See also: Spent nuclear fuel and High level waste
Long-lived radioactive waste from the back end of the fuel cycle is
especially relevant when designing a complete waste management plan for spent nuclear fuel
(SNF). When looking at long term radioactive decay, the actinides in
the SNF have a significant influence due to their characteristically
long half-lives. Depending on what a nuclear reactor is fueled with, the actinide composition in the SNF will be different.
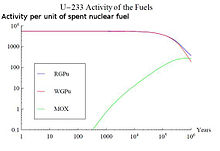
An example of this effect is the use of nuclear fuels with thorium.
Th-232 is a fertile material that can undergo a neutron capture
reaction and two beta minus decays, resulting in the production of
fissile U-233. The SNF of a cycle with thorium will contain U-233. Its radioactive decay will strongly influence the long-term activity
curve of the SNF around 1 million years. A comparison of the activity
associated to U-233 for three different SNF types can be seen in the
figure on the top right.
The burnt fuels are thorium with reactor-grade plutonium (RGPu), thorium with weapons-grade plutonium (WGPu) and Mixed Oxide fuel
(MOX). For RGPu and WGPu, the initial amount of U-233 and its decay
around 1 million years can be seen. This has an effect in the total
activity curve of the three fuel types. The absence of U-233 and its
daughter products in the MOX fuel results in a lower activity in region 3
of the figure on the bottom right, whereas for RGPu and WGPu the curve
is maintained higher due to the presence of U-233 that has not fully
decayed.
The use of different fuels in nuclear reactors results in different SNF composition, with varying activity curves.
Proliferation concerns
See also: Nuclear proliferation and Reactor-grade plutonium
Since uranium and plutonium are nuclear weapons materials, there have been proliferation concerns. Ordinarily (in spent nuclear fuel), plutonium is reactor-grade plutonium. In addition to plutonium-239, which is highly suitable for building nuclear weapons, it contains large amounts of undesirable contaminants: plutonium-240, plutonium-241, and plutonium-238.
These isotopes are difficult to separate, and more cost-effective ways
of obtaining fissile material exist (e.g. uranium enrichment or
dedicated plutonium production reactors).[16]
High-level waste is full of highly radioactive fission products, most of which are relatively short-lived. This is a concern since if the waste is stored, perhaps in deep geological storage,
over many years the fission products decay, decreasing the
radioactivity of the waste and making the plutonium easier to access.
The undesirable contaminant Pu-240 decays faster than the Pu-239, and
thus the quality of the bomb material increases with time (although its
quantity decreases during that time as well). Thus, some have argued, as
time passes, these deep storage areas have the potential to become
"plutonium mines", from which material for nuclear weapons can be
acquired with relatively little difficulty. Critics of the latter idea
point out that the half-life of Pu-240 is 6,560 years and Pu-239 is
24,110 years, and thus the relative enrichment of one isotope to the
other with time occurs with a half-life of 9,000 years (that is, it
takes 9000 years for the fraction of Pu-240 in a sample of mixed
plutonium isotopes, to spontaneously decrease by half—a typical
enrichment needed to turn reactor-grade into weapons-grade Pu). Thus
"weapons grade plutonium mines" would be a problem for the very far
future (>9,000 years from now), so that there remains a great deal of
time for technology to advance to solve it.[citation needed]
Pu-239 decays to U-235 which is suitable for weapons and which has a very long half-life (roughly 109
years). Thus plutonium may decay and leave uranium-235. However, modern
reactors are only moderately enriched with U-235 relative to U-238, so
the U-238 continues to serve as a denaturation agent for any U-235 produced by plutonium decay.
One solution to this problem is to recycle the plutonium and use it as a fuel e.g. in fast reactors. In pyrometallurgical fast reactors, the separated plutonium and uranium are contaminated by actinides and cannot be used for nuclear weapons.
Nuclear weapons decommissioning
Waste from nuclear weapons decommissioning is unlikely to contain much beta or gamma activity other than tritium and americium.
It is more likely to contain alpha-emitting actinides such as Pu-239
which is a fissile material used in bombs, plus some material with much
higher specific activities, such as Pu-238 or Po.
In the past the neutron trigger for an atomic bomb tended to be beryllium and a high activity alpha emitter such as polonium; an alternative to polonium is Pu-238. For reasons of national security, details of the design of modern bombs are normally not released to the open literature.
Some designs might contain a radioisotope thermoelectric generator using Pu-238 to provide a long lasting source of electrical power for the electronics in the device.
It is likely that the fissile material of an old bomb which is due
for refitting will contain decay products of the plutonium isotopes used
in it, these are likely to include U-236
from Pu-240 impurities, plus some U-235 from decay of the Pu-239; due
to the relatively long half-life of these Pu isotopes, these wastes from
radioactive decay of bomb core material would be very small, and in any
case, far less dangerous (even in terms of simple radioactivity) than
the Pu-239 itself.
The beta decay of Pu-241 forms Am-241;
the in-growth of americium is likely to be a greater problem than the
decay of Pu-239 and Pu-240 as the americium is a gamma emitter
(increasing external-exposure to workers) and is an alpha emitter which
can cause the generation of heat. The plutonium could be separated from the americium by several different processes; these would include pyrochemical processes and aqueous/organic solvent extraction. A truncated PUREX
type extraction process would be one possible method of making the
separation. Naturally occurring uranium is not fissile because it
contains 99.3% of U-238 and only 0.7% of U-235.
Legacy waste
Due to historic activities typically related to radium industry,
uranium mining, and military programs, there are numerous sites that
contain or are contaminated with radioactivity. In the United States
alone, the Department of Energy states there are "millions of gallons of radioactive waste" as well as "thousands of tons of spent nuclear fuel and material" and also "huge quantities of contaminated soil and water."[17]
Despite copious quantities of waste, the DOE has stated a goal of
cleaning all presently contaminated sites successfully by 2025.[17] The Fernald, Ohio
site for example had "31 million pounds of uranium product", "2.5
billion pounds of waste", "2.75 million cubic yards of contaminated soil
and debris", and a "223 acre portion of the underlying Great Miami
Aquifer had uranium levels above drinking standards."[17]
The United States has at least 108 sites designated as areas that are
contaminated and unusable, sometimes many thousands of acres.[17][18]
DOE wishes to clean or mitigate many or all by 2025, however the task
can be difficult and it acknowledges that some may never be completely
remediated. In just one of these 108 larger designations, Oak Ridge National Laboratory,
there were for example at least "167 known contaminant release sites"
in one of the three subdivisions of the 37,000-acre (150 km2) site.[17]
Some of the U.S. sites were smaller in nature, however, cleanup issues
were simpler to address, and DOE has successfully completed cleanup, or
at least closure, of several sites.[17]
Medical
Radioactive medical waste tends to contain beta particle and gamma ray emitters. It can be divided into two main classes. In diagnostic nuclear medicine a number of short-lived gamma emitters such as technetium-99m
are used. Many of these can be disposed of by leaving it to decay for a
short time before disposal as normal waste. Other isotopes used in
medicine, with half-lives in parentheses, include:
- Y-90, used for treating lymphoma (2.7 days)
- I-131, used for thyroid function tests and for treating thyroid cancer (8.0 days)
- Sr-89, used for treating bone cancer, intravenous injection (52 days)
- Ir-192, used for brachytherapy (74 days)
- Co-60, used for brachytherapy and external radiotherapy (5.3 years)
- Cs-137, used for brachytherapy, external radiotherapy (30 years)
Industrial
Industrial source waste can contain alpha, beta, neutron or gamma emitters. Gamma emitters are used in radiography while neutron emitting sources are used in a range of applications, such as oil well logging.[19]
Naturally occurring radioactive material (NORM)
Annual release of uranium and thorium radioisotopes from coal combustion, predicted by ORNL
to cumulatively amount to 2.9 million tons over the 1937-2040 period,
from the combustion of an estimated 637 billion tons of coal worldwide.[20]
Substances containing natural radioactivity are known as NORM.
After human processing that exposes or concentrates this natural
radioactivity (such as mining bringing coal to the surface or burning it
to produce concentrated ash), it becomes technologically-enhanced
naturally-occurring radioactive material (TENORM).[21] A lot of this waste is alpha particle-emitting matter from the decay chains of uranium and thorium. The main source of radiation in the human body is potassium-40 (40K), typically 17 milligrams in the body at a time and 0.4 milligrams/day intake.[22] Most rocks, due to their components, have a low level of radioactivity. Usually ranging from 1 milli-Sievert to 13 milli-Sievert
(mSv) annually depending on location, average radiation exposure from
natural radioisotopes is 2.0 mSv per person a year worldwide.[23] This makes up the majority of typical total dosage (with mean annual exposure from other sources amounting to 0.4 mSv from cosmic rays, 0.007 mSv from the legacy of past atmospheric nuclear testing along with the Chernobyl disaster,
0.0002 mSv from the nuclear fuel cycle, and, averaged over the whole
populace, 0.6 mSv medical tests and 0.005 mSv occupational exposure).[23]
TENORM is not regulated as restrictively as nuclear reactor waste,
though there are no significant differences in the radiological risks of
these materials.[24]
Coal
Coal
contains a small amount of radioactive uranium, barium, thorium and
potassium, but, in the case of pure coal, this is significantly less
than the average concentration of those elements in the Earth's crust.
The surrounding strata, if shale or mudstone, often contain slightly
more than average and this may also be reflected in the ash content of
'dirty' coals.[20][25] The more active ash minerals become concentrated in the fly ash precisely because they do not burn well.[20] The radioactivity of fly ash is about the same as black shale and is less than phosphate rocks, but is more of a concern because a small amount of the fly ash ends up in the atmosphere where it can be inhaled.[26] According to U.S. NCRP reports, population exposure from 1000-MWe power plants amounts to 490 person-rem/year for coal power plants, 100 times as great as nuclear power plants (4.8 person-rem/year during normal operation, or 136 person-rem/year for the complete nuclear fuel cycle).[20]
Oil and gas
Residues from the oil and gas
industry often contain radium and its decay products. The sulfate scale
from an oil well can be very radium rich, while the water, oil and gas
from a well often contain radon.
The radon decays to form solid radioisotopes which form coatings on the
inside of pipework. In an oil processing plant the area of the plant
where propane is processed is often one of the more contaminated areas of the plant as radon has a similar boiling point to propane.[27]
Classification of radioactive waste
Classifications of nuclear waste varies by country. The IAEA, which
publishes the Radioactive Waste Safety Standards (RADWASS), also plays a
significant role.[28]
Uranium tailings
Main article: Uranium tailings
Uranium tailings are waste by-product materials left over from the rough processing of uranium-bearing ore. They are not significantly radioactive. Mill tailings are sometimes referred to as 11(e)2 wastes, from the section of the Atomic Energy Act of 1946 that defines them. Uranium mill tailings typically also contain chemically hazardous heavy metal such as lead and arsenic. Vast mounds of uranium mill tailings are left at many old mining sites, especially in Colorado, New Mexico, and Utah.
See also: Uranium Mill Tailings Remedial Action
Low-level waste
Low level waste (LLW) is generated from hospitals and industry, as well as the nuclear fuel cycle. Low-level wastes include paper, rags, tools, clothing,
filters, and other materials which contain small amounts of mostly
short-lived radioactivity. Materials that originate from any region of
an Active Area are commonly designated as LLW as a precautionary measure
even if there is only a remote possibility of being contaminated with
radioactive materials. Such LLW typically exhibits no higher
radioactivity than one would expect from the same material disposed of
in a non-active area, such as a normal office block.
Some high-activity LLW requires shielding during handling and
transport but most LLW is suitable for shallow land burial. To reduce
its volume, it is often compacted or incinerated before disposal.
Low-level waste is divided into four classes: class A, class B, class C, and Greater Than Class C (GTCC).
Intermediate-level waste
Spent fuel flasks
are transported by railway in the United Kingdom. Each flask is
constructed of 14 in (360 mm) thick solid steel and weighs in excess of
50 tons
Intermediate-level waste (ILW) contains higher amounts of radioactivity and in some cases requires shielding. Intermediate-level wastes includes resins, chemical sludge and metal reactor nuclear fuel cladding, as well as contaminated materials from reactor decommissioning. It may be solidified in concrete or bitumen
for disposal. As a general rule, short-lived waste (mainly non-fuel
materials from reactors) is buried in shallow repositories, while
long-lived waste (from fuel and fuel reprocessing) is deposited in geological repository. U.S. regulations do not define this category of waste; the term is used in Europe and elsewhere.
High-level waste
High-level waste (HLW) is produced by nuclear reactors. It contains fission products and transuranic elements generated in the reactor core.
It is highly radioactive and often hot. HLW accounts for over 95
percent of the total radioactivity produced in the process of nuclear electricity generation. The amount of HLW worldwide is currently increasing by about 12,000 metric tons every year, which is the equivalent to about 100 double-decker buses or a two-story structure with a footprint the size of a basketball court.[29] A 1000-MW nuclear power plant produces about 27 tonnes of spent nuclear fuel (unreprocessed) every year.[30]
Transuranic waste
Transuranic waste (TRUW) as defined by U.S. regulations is, without regard to form or origin, waste that is contaminated with alpha-emitting transuranic radionuclides with half-lives greater than 20 years and concentrations greater than 100 nCi/g (3.7 MBq/kg),
excluding high-level waste. Elements that have an atomic number greater
than uranium are called transuranic ("beyond uranium"). Because of
their long half-lives, TRUW is disposed more cautiously than either low-
or intermediate-level waste. In the U.S., it arises mainly from weapons
production, and consists of clothing, tools, rags, residues, debris and
other items contaminated with small amounts of radioactive elements
(mainly plutonium).
Under U.S. law, transuranic waste is further categorized into
"contact-handled" (CH) and "remote-handled" (RH) on the basis of
radiation dose measured at the surface of the waste container. CH TRUW
has a surface dose rate not greater than 200 Roentgen equivalent man
per hour (two millisieverts/h), whereas RH TRUW has a surface dose rate
of 200 Röntgen equivalent man per hour (2 mSv/h) or greater. CH TRUW
does not have the very high radioactivity of high-level waste, nor its
high heat generation, but RH TRUW can be highly radioactive, with
surface dose rates up to 1 000 000 Röntgen equivalent man per hour
(10 000 mSv/h). The U.S. currently disposes of TRUW generated from
military facilities at the Waste Isolation Pilot Plant (WIPP) in a deep salt formation in New Mexico.[31]
Prevention of waste
A theoretical way to reduce waste accumulation is to phase out current reactors in favour of Generation IV Reactors or Liquid Fluoride Thorium Reactors, which output less waste per power generated. Fast reactors
can theoretically consume some existing waste, but the UK's Nuclear
Decommissioning Authority described this technology as immature and
commercially unproven, and unlikely to start before 2050.[32]
Management of waste
See also: High-level radioactive waste management, List of nuclear waste treatment technologies, and Environmental effects of nuclear power
Of particular concern in nuclear waste management are two long-lived
fission products, Tc-99 (half-life 220,000 years) and I-129 (half-life
15.7 million years), which dominate spent fuel radioactivity after a few
thousand years. The most troublesome transuranic elements in spent fuel
are Np-237 (half-life two million years) and Pu-239 (half-life 24,000
years).[33] Nuclear waste requires sophisticated treatment and management to successfully isolate it from interacting with the biosphere.
This usually necessitates treatment, followed by a long-term management
strategy involving storage, disposal or transformation of the waste
into a non-toxic form.[34]
Governments around the world are considering a range of waste
management and disposal options, though there has been limited progress
toward long-term waste management solutions.[35]
In second half of 20th century, several methods of disposal of radioactive waste were investigated by nuclear nations.[36] Which are;
- "Long term above ground storage", not implemented.
- "Disposal in outer space", not implemented.
- "Deep borehole disposal", not implemented.
- "Rock-melting", not implemented.
- "Disposal at subduction zones", not implemented.
- "Ocean disposal", done by USSR, UK, Switzerland, USA, Belgium, France, The Netherlands, Japan, Sweden, Russia, Germany, Italy and South Korea. (1954–93) This is no longer permitted by international agreements.
- "Sub seabed disposal", not implemented, not permitted by international agreements.
- "Disposal in ice sheets", rejected in Antarctic Treaty
- "Direct injection", done by USSR and USA.
Initial treatment of waste
Vitrification
Long-term storage of radioactive waste requires the stabilization of
the waste into a form which will neither react nor degrade for extended
periods of time. One way to do this is through vitrification.[37] Currently at Sellafield the high-level waste (PUREX first cycle raffinate) is mixed with sugar and then calcined. Calcination
involves passing the waste through a heated, rotating tube. The
purposes of calcination are to evaporate the water from the waste, and
de-nitrate the fission products to assist the stability of the glass
produced.[38]
The 'calcine' generated is fed continuously into an induction heated furnace with fragmented glass.[39]
The resulting glass is a new substance in which the waste products are
bonded into the glass matrix when it solidifies. This product, as a
melt, is poured into stainless steel
cylindrical containers ("cylinders") in a batch process. When cooled,
the fluid solidifies ("vitrifies") into the glass. Such glass, after
being formed, is highly resistant to water.[40]
After filling a cylinder, a seal is welded
onto the cylinder. The cylinder is then washed. After being inspected
for external contamination, the steel cylinder is stored, usually in an
underground repository. In this form, the waste products are expected to
be immobilized for thousands of years.[41]
The glass inside a cylinder is usually a black glossy substance. All this work (in the United Kingdom) is done using hot cell systems. The sugar is added to control the ruthenium chemistry and to stop the formation of the volatile RuO4 containing radioactive ruthenium isotopes. In the West, the glass is normally a borosilicate glass (similar to Pyrex), while in the former Soviet bloc it is normal to use a phosphate glass.[42] The amount of fission products in the glass must be limited because some (palladium, the other Pt group metals, and tellurium)
tend to form metallic phases which separate from the glass. Bulk
vitrification uses electrodes to melt soil and wastes, which are then
buried underground.[43]
In Germany a vitrification plant is in use; this is treating the waste
from a small demonstration reprocessing plant which has since been
closed down.[38][44]
Ion exchange
It is common for medium active wastes in the nuclear industry to be treated with ion exchange
or other means to concentrate the radioactivity into a small volume.
The much less radioactive bulk (after treatment) is often then
discharged. For instance, it is possible to use a ferric hydroxide floc to remove radioactive metals from aqueous mixtures.[45]
After the radioisotopes are absorbed onto the ferric hydroxide, the
resulting sludge can be placed in a metal drum before being mixed with
cement to form a solid waste form.[46] In order to get better long-term performance (mechanical stability) from such forms, they may be made from a mixture of fly ash, or blast furnace slag, and Portland cement, instead of normal concrete (made with Portland cement, gravel and sand).
Synroc
The Australian Synroc
(synthetic rock) is a more sophisticated way to immobilize such waste,
and this process may eventually come into commercial use for civil
wastes (it is currently being developed for US military wastes). Synroc
was invented by the late Prof Ted Ringwood (a geochemist) at the Australian National University.[47] The Synroc contains pyrochlore
and cryptomelane type minerals. The original form of Synroc (Synroc C)
was designed for the liquid high level waste (PUREX raffinate) from a light water reactor. The main minerals in this Synroc are hollandite (BaAl2Ti6O16), zirconolite (CaZrTi2O7) and perovskite (CaTiO3). The zirconolite and perovskite are hosts for the actinides. The strontium and barium will be fixed in the perovskite. The caesium will be fixed in the hollandite.
Long term management of waste
The time frame in question when dealing with radioactive waste ranges from 10,000 to 1,000,000 years,[48] according to studies based on the effect of estimated radiation doses.[49] Researchers suggest that forecasts of health detriment for such periods should be examined critically.[50] [51] Practical studies only consider up to 100 years as far as effective planning[52] and cost evaluations[53] are concerned. Long term behavior of radioactive wastes remains a subject for ongoing research projects in geoforecasting.[54]
Above-ground disposal
Dry cask storage typically involves taking waste from a spent fuel pool and sealing it (along with an inert gas) in a steel cylinder, which is placed in a concrete
cylinder which acts as a radiation shield. It is a relatively
inexpensive method which can be done at a central facility or adjacent
to the source reactor. The waste can be easily retrieved for
reprocessing.[55]
Geologic disposal
The process of selecting appropriate deep final repositories
for high level waste and spent fuel is now under way in several
countries with the first expected to be commissioned some time after
2010. The basic concept is to locate a large, stable geologic formation
and use mining technology to excavate a tunnel, or large-bore tunnel boring machines (similar to those used to drill the Channel Tunnel
from England to France) to drill a shaft 500 metres (1,600 ft) to 1,000
metres (3,300 ft) below the surface where rooms or vaults can be
excavated for disposal of high-level radioactive waste. The goal is to
permanently isolate nuclear waste from the human environment. Many
people remain uncomfortable with the immediate stewardship cessation of this disposal system, suggesting perpetual management and monitoring would be more prudent.
Because some radioactive species have half-lives longer than one
million years, even very low container leakage and radionuclide
migration rates must be taken into account.[56]
Moreover, it may require more than one half-life until some nuclear
materials lose enough radioactivity to cease being lethal to living
things. A 1983 review of the Swedish radioactive waste disposal program
by the National Academy of Sciences found that country’s estimate of
several hundred thousand years—perhaps up to one million years—being
necessary for waste isolation “fully justified.”[57] Aside from dilution, chemically toxic stable elements in some waste such as arsenic remain toxic for up to billions of years or indefinitely.[58]
Sea-based options for disposal of radioactive waste[59]
has been suggested by the finding that deep waters in the North
Atlantic Ocean do not present an exchange with shallow waters for about
140 years based on oxygen content data recorded over a period of 25
years.[60] They include burial beneath a stable abyssal plain, burial in a subduction zone that would slowly carry the waste downward into the Earth's mantle,[61][62]
and burial beneath a remote natural or human-made island. While these
approaches all have merit and would facilitate an international solution
to the problem of disposal of radioactive waste, they would require an amendment of the Law of the Sea.[63]
Article 1 (Definitions), 7., of the 1996 Protocol to the Convention
on the Prevention of Marine Pollution by Dumping of Wastes and Other
Matter, (the London Dumping Convention) states:
- “Sea” means all marine waters other than the internal waters of States, as well as the seabed and the subsoil thereof; it does not include sub-seabed repositories accessed only from land.”
The proposed land-based subductive waste disposal method disposes of nuclear waste in a subduction zone accessed from land,[64]
and therefore is not prohibited by international agreement. This method
has been described as the most viable means of disposing of radioactive
waste,[65] and as the state-of-the-art as of 2001 in nuclear waste disposal technology.[66] Another approach termed Remix & Return[67] would blend high-level waste with uranium mine and mill tailings down to the level of the original radioactivity of the uranium ore,
then replace it in inactive uranium mines. This approach has the merits
of providing jobs for miners who would double as disposal staff, and of
facilitating a cradle-to-grave cycle for radioactive materials, but
would be inappropriate for spent reactor fuel in the absence of
reprocessing, due to the presence in it of highly toxic radioactive
elements such as plutonium.
Deep borehole disposal
is the concept of disposing of high-level radioactive waste from
nuclear reactors in extremely deep boreholes. Deep borehole disposal
seeks to place the waste as much as 5 kilometres (3.1 mi) beneath the
surface of the Earth and relies primarily on the immense natural
geological barrier to confine the waste safely and permanently so that
it should never pose a threat to the environment. The Earth's crust
contains 120 trillion tons of thorium and 40 trillion tons of uranium
(primarily at relatively trace concentrations of parts per million each adding up over the crust's 3 * 1019 ton mass), among other natural radioisotopes.[68][69][70]
Since the fraction of nuclides decaying per unit of time is inversely
proportional to an isotope's half-life, the relative radioactivity of
the lesser amount of human-produced radioisotopes (thousands of tons
instead of trillions of tons) would diminish once the isotopes with far
shorter half-lives than the bulk of natural radioisotopes decayed.
In January 2013, Cumbria county council rejected UK central government proposals to start work on an underground storage dump for nuclear waste near to the Lake District National Park.
"For any host community, there will be a substantial community benefits
package and worth hundreds of millions of pounds" said Ed Davey, Energy
Secretary, but nonetheless, the local elected body voted 7-3 against
research continuing, after hearing evidence from independent geologists
that "the fractured strata of the county was impossible to entrust with
such dangerous material and a hazard lasting millennia."[71][72]
Transmutation
Main article: Nuclear transmutation
There have been proposals for reactors that consume nuclear waste and
transmute it to other, less-harmful nuclear waste. In particular, the Integral Fast Reactor
was a proposed nuclear reactor with a nuclear fuel cycle that produced
no transuranic waste and in fact, could consume transuranic waste. It
proceeded as far as large-scale tests, but was then canceled by the US
Government. Another approach, considered safer but requiring more
development, is to dedicate subcritical reactors to the transmutation of the left-over transuranic elements.
An isotope that is found in nuclear waste and that represents a
concern in terms of proliferation is Pu-239. The estimated world total
of plutonium in the year 2000 was of 1,645 MT, of which 210 MT had been
separated by reprocessing. The large stock of plutonium is a result of
its production inside uranium-fueled reactors and of the reprocessing of
weapons-grade plutonium during the weapons program. An option for
getting rid of this plutonium is to use it as a fuel in a traditional
Light Water Reactor (LWR). Several fuel types with differing plutonium
destruction efficiencies are under study. See Nuclear transmutation.
Transmutation was banned in the US in April 1977 by President Carter due to the danger of plutonium proliferation,[73] but President Reagan rescinded the ban in 1981.[74]
Due to the economic losses and risks, construction of reprocessing
plants during this time did not resume. Due to high energy demand, work
on the method has continued in the EU. This has resulted in a practical nuclear research reactor called Myrrha in which transmutation is possible. Additionally, a new research program called ACTINET has been started in the EU
to make transmutation possible on a large, industrial scale. According
to President Bush's Global Nuclear Energy Partnership (GNEP) of 2007,
the US is now actively promoting research on transmutation technologies
needed to markedly reduce the problem of nuclear waste treatment.[75]
There have also been theoretical studies involving the use of fusion reactors as so called "actinide burners" where a fusion reactor plasma such as in a tokamak,
could be "doped" with a small amount of the "minor" transuranic atoms
which would be transmuted (meaning fissioned in the actinide case) to
lighter elements upon their successive bombardment by the very high
energy neutrons produced by the fusion of deuterium and tritium in the reactor. A study at MIT found that only 2 or 3 fusion reactors with parameters similar to that of the International Thermonuclear Experimental Reactor (ITER) could transmute the entire annual minor actinide production from all of the light water reactors presently operating in the United States fleet while simultaneously generating approximately 1 gigawatt of power from each reactor.[76]
Re-use of waste
Main article: Nuclear reprocessing
Another option is to find applications for the isotopes in nuclear waste so as to re-use them.[77] Already, caesium-137, strontium-90 and a few other isotopes are extracted for certain industrial applications such as food irradiation and radioisotope thermoelectric generators. While re-use does not eliminate the need to manage radioisotopes, it reduces the quantity of waste produced.
The Nuclear Assisted Hydrocarbon Production Method,[78]
Canadian patent application 2,659,302, is a method for the temporary or
permanent storage of nuclear waste materials comprising the placing of
waste materials into one or more repositories or boreholes constructed
into an unconventional oil
formation. The thermal flux of the waste materials fracture the
formation, alters the chemical and/or physical properties of hydrocarbon
material within the subterranean formation to allow removal of the
altered material. A mixture of hydrocarbons, hydrogen, and/or other
formation fluids are produced from the formation. The radioactivity of
high-level radioactive waste affords proliferation resistance to
plutonium placed in the periphery of the repository or the deepest
portion of a borehole.
Breeder reactors
can run on U-238 and transuranic elements, which comprise the majority
of spent fuel radioactivity in the 1000-100000 year time span.
Space disposal
Space disposal is attractive because it permanently removes nuclear
waste from the environment. It has significant disadvantages, such as
the potential for catastrophic failure of a launch vehicle,
which could spread radioactive material into the atmosphere and around
the world. A high number of launches would be required because no
individual rocket would be able to carry very much of the material
relative to the total amount that needs to be disposed of. This makes
the proposal impractical economically and it increases the risk of at
least one or more launch failures.[79] To further complicate matters, international agreements on the regulation of such a program would need to be established.[80]
Costs and inadequate reliability of modern rocket launch systems for
space disposal has been one of the motives for interest in non-rocket space launch systems such as mass drivers, space elevators, and other proposals.
National management plans
See also: High-level radioactive waste management
Most countries are considerably ahead of the United States in
developing plans for high-level radioactive waste disposal. Sweden and
Finland are furthest along in committing to a particular disposal
technology, while many others reprocess spent fuel or contract with
France or Great Britain to do it, taking back the resulting plutonium
and high-level waste. “An increasing backlog of plutonium from
reprocessing is developing in many countries... It is doubtful that
reprocessing makes economic sense in the present environment of cheap
uranium.”[81]
In many European countries (e.g., Britain, Finland, the Netherlands,
Sweden and Switzerland) the risk or dose limit for a member of the
public exposed to radiation from a future high-level nuclear waste
facility is considerably more stringent than that suggested by the
International Commission on Radiation Protection or proposed in the
United States. European limits are often more stringent than the
standard suggested in 1990 by the International Commission on Radiation
Protection by a factor of 20, and more stringent by a factor of ten than
the standard proposed by the US Environmental Protection Agency (EPA)
for Yucca Mountain nuclear waste repository for the first 10,000 years after closure.[82]
The U.S. EPA’s proposed standard for greater than 10,000 years is 250 times more permissive than the European limit.[82] The U.S. EPA proposed a legal limit of a maximum of 3.5 milli-Sieverts (350 millirem)
each annually to local individuals after 10,000 years, which would be
up to several percent of the exposure currently received by some
populations in the highest natural background regions on Earth, though the U.S. DOE predicted that received dose would be much below that limit.[83]
Over a timeframe of thousands of years, after the most active short
half-life radioisotopes decayed, burying U.S. nuclear waste would
increase the radioactivity in the top 2000 feet of rock and soil in the United States (10 million km2) by ≈ 1 part in 10 million over the cumulative amount of natural radioisotopes
in such a volume, but the vicinity of the site would have a far higher
concentration of artificial radioisotopes underground than such an
average.[84]
Mongolia
After serious opposition had risen about plans and negotiations between Mongolia
with Japan and the United States of America to build nuclear waste
facilities in Mongolia, Mongolia stopped all negotiations in September
2011. These negotiations started after U.S. Deputy Secretary of Energy Daniel B. Poneman
visited Mongolia in September, 2010. Talks were held in Washington DC
between officials of Japan, the United States and Mongolia in February
2011. After this the United Arab Emirates
(UAE), which wanted to buy nuclear fuel from Mongolia, joined in the
negotiations. The talks were kept secret, and although The Mainichi Daily News
reported on it in May, Mongolia officially denied the existence of
these negotiations. But alarmed by this news, Mongolian citizens
protested against the plans, and demanded the government withdraw the
plans and disclose information. The Mongolian President Tsakhia Elbegdorj
issued a presidential order on Sept. 13 banning all negotiations with
foreign governments or international organizations on nuclear waste
storage plans in Mongolia.[85]
The Mongolian government has accused the newspaper of distributing
false claims around the world. After the presidential order, the
Mongolian president fired the individual that was supposedly involved in
these conversations.
Illegal dumping
Main article: Toxic waste dumping by the 'Ndrangheta
Authorities in Italy are investigating a 'Ndrangheta mafia clan accused of trafficking and illegally dumping nuclear waste. According to a turncoat, a manager of the Italy’s state energy research agency Enea
paid the clan to get rid of 600 drums of toxic and radioactive waste
from Italy, Switzerland, France, Germany, and the US, with Somalia
as the destination, where the waste was buried after buying off local
politicians. Former employees of Enea are suspected of paying the
criminals to take waste off their hands in the 1980s and 1990s.
Shipments to Somalia continued into the 1990s, while the 'Ndrangheta
clan also blew up shiploads of waste, including radioactive hospital
waste, and sending them to the sea bed off the Calabrian coast.[86] According to the environmental group Legambiente, former members of the 'Ndrangheta have said that they were paid to sink ships with radioactive material for the last 20 years.[87]
Accidents involving radioactive waste
Main article: Nuclear and radiation accidents
A few incidents have occurred when radioactive material was disposed
of improperly, shielding during transport was defective, or when it was
simply abandoned or even stolen from a waste store.[88] In the Soviet Union, waste stored in Lake Karachay was blown over the area during a dust storm after the lake had partly dried out.[89] At Maxey Flat, a low-level radioactive waste facility located in Kentucky,
containment trenches covered with dirt, instead of steel or cement,
collapsed under heavy rainfall into the trenches and filled with water.
The water that invaded the trenches became radioactive and had to be
disposed of at the Maxey Flat
facility itself. In other cases of radioactive waste accidents, lakes
or ponds with radioactive waste accidentally overflowed into the rivers
during exceptional storms.[citation needed] In Italy, several radioactive waste deposits let material flow into river water, thus contaminating water for domestic use.[90] In France, in the summer of 2008 numerous incidents happened;[91] in one, at the Areva plant in Tricastin,
it was reported that during a draining operation, liquid containing
untreated uranium overflowed out of a faulty tank and about 75 kg of the
radioactive material seeped into the ground and, from there, into two
rivers nearby;[92] in another case, over 100 staff were contaminated with low doses of radiation.[93]
Scavenging of abandoned radioactive material has been the cause of several other cases of radiation exposure, mostly in developing nations,
which may have less regulation of dangerous substances (and sometimes
less general education about radioactivity and its hazards) and a market
for scavenged goods and scrap metal. The scavengers and those who buy
the material are almost always unaware that the material is radioactive
and it is selected for its aesthetics or scrap value.[94]
Irresponsibility on the part of the radioactive material's owners,
usually a hospital, university or military, and the absence of
regulation concerning radioactive waste, or a lack of enforcement of
such regulations, have been significant factors in radiation exposures.
For an example of an accident involving radioactive scrap originating
from a hospital see the Goiânia accident.[94]
Transportation accidents involving spent nuclear fuel from power
plants are unlikely to have serious consequences due to the strength of
the spent nuclear fuel shipping casks.[95]
On 15 December 2011 top government spokesman Osamu Fujimura of the
Japanese government admitted that nuclear substances were found in the
waste of Japanese nuclear facilities. Although Japan did commit itself
in 1977 to these inspections in the safeguard agreement with the IAEA,
the reports were kept secret for the inspectors of the International Atomic Energy Agency.[citation needed]
Japan did start discussions with the IAEA about the large quantities of
enriched uranium and plutonium that were discovered in nuclear waste
cleared away by Japanese nuclear operators.[citation needed]
At the press conference Fujimura said: "Based on investigations so far,
most nuclear substances have been properly managed as waste, and from
that perspective, there is no problem in safety management," But
according to him, the matter was at that moment still being
investigated.[96]
See also
- Background radiation
- Deep borehole disposal
- Deep geological repository
- Depleted uranium
- Ducrete
- Environmental remediation
- Geomelting
- Hot cell
- Lists of nuclear disasters and radioactive incidents
- Mixed waste (radioactive/hazardous)
- Nuclear decommissioning
- Personal protective equipment
- Radiation protection
- Radioactive contamination
- Radioactive scrap metal
- Toxic waste
- Waste management
References
- Jump up ^ IAEA Joint Convention on Safety of Radioactive Waste
- Jump up ^ Nuclear Information and Resource Service, Radioactive Waste Project. Retrieved September 2007.
- Jump up ^ "What about Iodine-129 - Half-Life is 15 Million Years". Berkeley Radiological Air and Water Monitoring Forum. University of California. 28 March 2011. Retrieved 1 December 2012.
- Jump up ^ Attix, Frank (1986). Introduction to Radiological Physics and Radiation Dosimetry. New York: Wiley-VCH. pp. 2–15,468,474. ISBN 0-471-01146-0, 9780471011460 Check
|isbn=value (help). Retrieved 9-3-2011. - Jump up ^ Anderson, Mary; Woessner, William (1992). Applied Groundwater Modeling. San Diego, CA: Academic Press Inc. pp. 325–327. ISBN 0-12-059485-4. Retrieved 9-3-2011.
- Jump up ^ Plus radium (element 88). While actually a sub-actinide, it immediately precedes actinium (89) and follows a three element gap of instability after polonium (84) where no isotopes have half-lives of at least four years (the longest-lived isotope in the gap is radon-222 with a half life of less than four days). Radium's longest lived isotope, at a notable 1600 years, thus merits the element's inclusion here.
- Jump up ^ Specifically from thermal neutron fission of U-235, e.g. in a typical nuclear reactor.
- Jump up ^ This is the heaviest isotope with a half-life of at least four years before the "Sea of Instability".
- Jump up ^ Excluding those 'classically stable' isotopes with half-lives significantly in excess of 232Th, e.g. while 113mCd has a half-life of only fourteen years, that of 113Cd is nearly eight quadrillion.
- Jump up ^ Goldstein, Inge, and Martin Goldstein. How much risk? Oxford University Press, 2002. ISBN 0-19-513994-1
- Jump up ^ Gofman, John W. Radiation and human health. San Francisco: Sierra Club Books, 1981, 787.
- Jump up ^ Gofman, John W. Radiation and human health. San Francisco: Sierra Club Books, 1981, 760-848.
- Jump up ^ Cochran, Robert; Tsoulfanidis (1999). The Nuclear Fuel Cycle: Analysis and Management. La Grange Park, IL: American Nuclear Society. pp. 52–57. ISBN 0-89448-451-6. Retrieved 9-3-2011.
- Jump up ^ Depleted Uranium-FAQs, Janes
- Jump up ^ "Continuous Plutonium Recycling In India: Improvements in Reprocessing Technology".
- Jump up ^ World Nuclear Association (2009-03). "Plutonium". Retrieved 2010-03-18.
- ^ Jump up to: a b c d e f U.S. Department of Energy Environmental Management - "Department of Energy Five Year Plan FY 2007-FY 2011 Volume II." Retrieved 8 April 2007.
- Jump up ^ American Scientist Jan/Feb 2007
- Jump up ^ "Nuclear Logging". Retrieved 2009-07-07.
- ^ Jump up to: a b c d Coal Combustion - ORNL Review Vol. 26, No. 3&4, 1993
- Jump up ^ "TENORM Sources | Radiation Protection | US EPA". Epa.gov. 2006-06-28. Retrieved 2013-08-01.
- Jump up ^ Idaho State University. Radioactivity in Nature
- ^ Jump up to: a b United Nations Scientific Committee on the Effects of Atomic Radiation. Sources and Effects of Ionizing Radiation, UNSCEAR 2008
- Jump up ^ "Regulation of TENORM". Tenorm.com. Retrieved 2013-08-01.
- Jump up ^ Cosmic origins of Uranium
- Jump up ^ U.S. Geological Survey, Radioactive Elements in Coal and Fly Ash: Abundance, Forms, and Environmental Significance, Fact Sheet FS-163-1997, October 1997. Retrieved September 2007.
- Jump up ^ Survey & Identification of NORM Contaminated Equipment
- Jump up ^ http://www-pub.iaea.org/MTCD/publications/PDF/Pub950e_web.pdf
- Jump up ^ Marathon Resources Ltd :: Our Business :: Uranium Industry :: Nuclear Waste
- Jump up ^ Radioactive Waste management
- Jump up ^ Why WIPP?
- Jump up ^ Edwards, Rob (2012-01-24). "Plans for Sellafield plutonium reactor rejected". The Guardian.
- Jump up ^ Vandenbosch, Robert, and Susanne E. Vandenbosch. 2007. Nuclear waste stalemate. Salt Lake City: University of Utah Press, 21.
- Jump up ^ M. I. Ojovan, W.E. Lee. An Introduction to Nuclear Waste Immobilisation, Elsevier Science Publishers B.V., Amsterdam, 315pp. (2005)
- Jump up ^ See, for example, Paul Brown, 'Shoot it at the sun. Send it to Earth's core. What to do with nuclear waste?', The Guardian, 14 April 2004.
- Jump up ^ World Nuclear Association “Storage and Disposal Options” retrieved 2011-11-14
- Jump up ^ M. I. Ojovan, W.E. Lee. An Introduction to Nuclear Waste Immobilisation, Elsevier, Amsterdam, 315pp. (2005)
- ^ Jump up to: a b National Research Council (1996). Nuclear Wastes: Technologies for Separation and Transmutation. Washington DC: National Academy Press.
- Jump up ^ "Laboratory-scale vitrification and leaching of Hanford high-level waste for the purpose of simulant and glass property models validation". Retrieved 2009-07-07.
- Jump up ^ Ojovanm M.I. et al. (2006). "Corrosion of nuclear waste glasses in non-saturated conditions: Time-Temperature behaviour" (PDF). Retrieved 2008-06-30.
- Jump up ^ OECD Nuclear Energy Agency (1994). The Economics of the Nuclear Fuel Cycle. Paris: OECD Nuclear Energy Agency.
- Jump up ^ M.I. Ojovan, W.E. Lee. Glassy wasteforms for nuclear waste immobilisation. Metallurgical and Materials Transactions A, 42 (4), 837-851 (2011).
- Jump up ^ "Waste Form Release Calculations for the 2005 Integrated Disposal Facility Performance Assessment" (PDF). PNNL-15198. Pacific Northwest National Laboratory. July 2005. Retrieved 2006-11-08.
- Jump up ^ Hensing, I., and W. Schultz (1995). Economic Comparison of Nuclear Fuel Cycle Options. Cologne: Energiewirtschaftlichen Instituts.
- Jump up ^ Author: Marion Brünglinghaus, ENS, European Nuclear Society. "Waste processing". Euronuclear.org. Retrieved 2013-08-01.
- Jump up ^ Removal of Silicon from High Level Waste Streams via Ferric Flocculation
- Jump up ^ World Nuclear Association, Synroc, Nuclear Issues Briefing Paper 21. Retrieved January 2009.
- Jump up ^ National Research Council (1995). Technical Bases for Yucca Mountain Standards. Washington, D.C.: National Academy Press. cited in "The Status of Nuclear Waste Disposal". The American Physical Society. January 2006. Retrieved 2008-06-06.
- Jump up ^ "Public Health and Environmental Radiation Protection Standards for Yucca Mountain, Nevada; Proposed Rule" (PDF). Environmental Protection Agency. 2005-08-22. Retrieved 2008-06-06.
- Jump up ^ Peterson, Per; William Kastenberg and Michael Corradini. "Nuclear Waste and the Distant Future". Issues in Science and Technology (Washington, DC: National Academy of Sciences) (Summer 2006).
- Jump up ^ "Issues relating to safety standards on the geological disposal of radioactive waste" (PDF). International Atomic Energy Agency. 2001-06-22. Retrieved 2008-06-06.
- Jump up ^ "IAEA Waste Management Database: Report 3 - L/ILW-LL" (PDF). International Atomic Energy Agency. 2000-03-28. Retrieved 2008-06-06.
- Jump up ^ "Decommissioning costs of WWER-440 nuclear power plants" (PDF). International Atomic Energy Agency. November 2002. Retrieved 2008-06-06.
- Jump up ^ International Atomic Energy Agency, Spent Fuel and High Level Waste: Chemical Durability and Performance under Simulated Repository Conditions, IAEA-TECDOC-1563, October 2007.
- Jump up ^ "Fact Sheet on Dry Cask Storage of Spent Nuclear Fuel". NRC. May 7, 2009. Retrieved 2011-06-25.
- Jump up ^ Vandenbosch, Robert, and Susanne E. Vandenbosch. 2007. Nuclear waste stalemate. Salt Lake City: University of Utah Press, 10.
- Jump up ^ Yates, Marshall. 1989. “DOE waste management criticized: On-site storage urged.” Public Utilities Fortnightly 124 (July 6): 33.
- Jump up ^ [Hazards of High-Level Radioactive Waste http://www.phyast.pitt.edu/~blc/book/chapter11.html]
- Jump up ^ Sea-based Nuclear Waste Solutions
- Jump up ^ J.P. Hoare Electrochemistry of Oxygen, Interscience Publishers, 1968
- Jump up ^ Hafemeister, David W. (2007). Physics of societal issues: calculations on national security, environment, and energy. Berlin: Springer. ISBN 0-387-95560-7., p. 187.
- Jump up ^ Shipman, J.T.; Wison J.D. & Todd A. (2007). An Introduction to Physical Science (10 ed.). Cengage Learning. p. 279. ISBN 978-0-618-93596-3.
- Jump up ^ "Dumping and Loss overview". Oceans in the Nuclear Age. Retrieved March 23, 2011.
- Jump up ^ Subductive Waste Disposal Method
- Jump up ^ Utah Nuclear Waste Summary, by Tricia Jack, Jordan Robertson, Center for Public Policy & Administration, University of Utah
- Jump up ^ Radioactive waste: The problem and its management, by K. R. Rao, Current science, vol. 81, no. 12, 25 december 2001
- Jump up ^ Remix & Return
- Jump up ^ Sevior M. (2006). "Considerations for nuclear power in Australia" (PDF). International Journal of Environmental Studies 63 (6): 859–872. doi:10.1080/00207230601047255.
- Jump up ^ Thorium Resources In Rare Earth Elements
- Jump up ^ American Geophysical Union, Fall Meeting 2007, abstract #V33A-1161. Mass and Composition of the Continental Crust
- Jump up ^ Wainwright, Martin (30 January 2013). "Cumbria rejects underground nuclear storage dump". The Guardian. Retrieved 1 February 2013.
- Jump up ^ Macalister, Terry (31 January 2013). "Cumbria sticks it to the nuclear dump lobby – despite all the carrots on offer". The Guardian. Retrieved 1 February 2013.
- Jump up ^ Transmutation being banned in the US since 1977
- Jump up ^ National Policy Analysis #396: The Separations Technology and Transmutation Systems (STATS) Report: Implications for Nuclear Power Growth and Energy Sufficiency - February 2002
- Jump up ^ http://www.gnep.energy.gov/pdfs/GNEP_SOP.pdf
- Jump up ^ Jeffrey P. Freidberg. "Department of Nuclear Engineering: Reports to the President 2000-2001". Web.mit.edu. Retrieved 2013-08-01.
- Jump up ^ http://www.heritage.org/Research/EnergyandEnvironment/upload/86845_1.pdf
- Jump up ^ "酵素でプチ断食|成功させる秘訣は代替ドリンクにあった!". Nuclearhydrocarbons.com. Retrieved 2013-08-01.
- Jump up ^ National Research Council (U.S.). Committee on Disposition of High-Level Radioactive Waste Through Geological Isolation (2001). Disposition of high-level waste and spent nuclear fuel: the continuing societal and technical challenges. National Academies Press. p. 122. ISBN 978-0-309-07317-2.
- Jump up ^ "Managing nuclear waste: Options considered". DOE Factsheets. Department of Energy: Office of Civilian Radioactive Waste Management, Yucca Mountain Project. November 2003. Archived from the original on 2009-05-15.
- Jump up ^ Vandenbosch, Robert, and Susanne E. Vandenbosch. 2007. Nuclear waste stalemate. Salt Lake City: University of Utah Press, 247.
- ^ Jump up to: a b Vandenbosch, Robert, and Susanne E. Vandenbosch. 2007. Nuclear waste stalemate. Salt Lake City: University of Utah Press, 248.
- Jump up ^ U.S. Federal Register. 40 CFR Part 197. Environmental Protection Agency. Public Health and Environmental Radiation Protection Standards for Yucca Mountain, Nevada; Final Rule
- Jump up ^ Interdisciplinary Science Reviews 23:193-203;1998. Dr. Bernard L. Cohen, University of Pittsburgh. Perspectives on the High Level Waste Disposal Problem
- Jump up ^ The Mainichi Daily News (15 October 2011)Mongolia abandons nuclear waste storage plans, and informs Japan of decision
- Jump up ^ From cocaine to plutonium: mafia clan accused of trafficking nuclear waste, The Guardian, October 9, 2007
- Jump up ^ Mafia sank boat with radioactive waste: official, AFP, September 14, 2009
- Jump up ^ Strengthening the safety of radiation sources & the security of radioactive materials: timely action, by Abel J.González, IAEA Bulletin, 41/3/1999
- Jump up ^ GlobalSecurity.org, Chelyabinsk-65/Ozersk. Retrieved September 2007.
- Jump up ^ Report RAI.it, L'Eredità (in Italian), 2 November 2008
- Jump up ^ Reuters UK, New incident at French nuclear plant. Retrieved March 2009.
- Jump up ^ "'It feels like a sci-fi film' - accidents tarnish nuclear dream". The Guardian. 25 July 2008.
- Jump up ^ Reuters UK, Too many French nuclear workers contaminated. Retrieved March 2009.
- ^ Jump up to: a b International Atomic Energy Agency, The radiological accident in Goiânia, 1988. Retrieved September 2007.
- Jump up ^ "Nuclear Flask Train Crash Test - BBC News 1984". YouTube. 1984-07-17. Retrieved 2013-08-01.
- Jump up ^ The Mainichi Daily News) December 15, 2011 )Gov't admits nuclear substances found in waste, unreported to IAEA
Further reading
- Babu, B.V., and S. Karthik, Energy Education Science and Technology, 2005, 14, 93–102. An overview of waste from the nuclear fuel cycle.
- Bedinger, M.S. (1989). Geohydrologic aspects for siting and design of low-level radioactive-waste disposal [U.S. Geological Survey Circular 1034]. Washington, D.C.: U.S. Department of the Interior, U.S. Geological Survey.
- Fentiman, Audeen W. and James H. Saling. Radioactive Waste Management. New York: Taylor & Francis, 2002. Second ed.
- Hamblin, Jacob Darwin (2008). Poison in the Well: Radioactive Waste in the Oceans at the Dawn of the Nuclear Age. Piscataway, NJ: Rutgers University Press.
- Hewitt, Robin (1985). Outer Space: the Easy Way Out?, Sierra Club Radioactive Waste Campaign, N.Y., NY, 1985. ([1]).
- Marshall, Alan (2005) The Social and Ethical Aspects of Nuclear Waste, Electronic Green Journal 21, 1.
- Marshall, Alan. (2005) Questioning the Motivations for International Repositories for Nuclear Waste Global Environmental Politics, Volume 5, Number 2, May 2005, pp. 1–9
- Marshall, Alan. (2006) Dangerous Dawn: The New Nuclear Age, FoE and BNI, Melbourne.
- Marshall, Alan (2007) Questioning Nuclear Waste Substitution: A Case Study. Science and Engineering Ethics 13 (1).
- Marshall, Alan. (2008) Leaving Messages about Our Radioactive Waste for Future Generations, in A. P Latiffer, ed, Nuclear Waste Research, Nova Publishers, pp37–46.
- Nuclear and Radiation Studies Board. (NRSB) Going the Distance? The Safe Transport of Spent Nuclear Fuel and High-Level Radioactive Waste in the United States ISBN 0-309-10004-6
- M.I. Ojovan (ed.). Handbook of advanced radioactive waste conditioning technologies. ISBN 1-84569-626-3. Oxford, 512 p. (2011). http://www.woodheadpublishing.com/6269
External links
- Alsos Digital Library - Radioactive Waste (annotated bibliography)
- Euridice European Interest Group in charge of Hades URL operation (link)
- Ondraf/Niras, the waste management authority in Belgium (link)
- Critical Hour: Three Mile Island, The Nuclear Legacy, And National Security (PDF)
- Environmental Protection Agency - Yucca Mountain (documents)
- Grist.org - How to tell future generations about nuclear waste (article)
- SmartPlanet.com - Ticking Time Bombs: What Should We Do With Nuclear Waste
- International Atomic Energy Agency - Internet Directory of Nuclear Resources (links)
- Nuclear Files.org - Yucca Mountain (documents)
- Nuclear Regulatory Commission - Radioactive Waste (documents)
- Nuclear Regulatory Commission - Spent Fuel Heat Generation Calculation (guide)
- Radwaste Solutions (magazine)
- UNEP Earthwatch - Radioactive Waste (documents and links)
- World Nuclear Association - Radioactive Waste (briefing papers)
- Worries can’t be buried as nuclear waste piles up, Los Angeles Times, January 21, 2008
- RadWaste.org






Tidak ada komentar:
Posting Komentar